Catalog No.
DHB74002
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
NF-HEV, IL-1F11, C9orf26, IL-33, Interleukin-1 family member 11, IL33, Nuclear factor from high endothelial venules, NFHEV, Interleukin-33, IL1F11
Concentration
1.3 mg/ml
Endotoxin level
Please contact with the lab for this information.
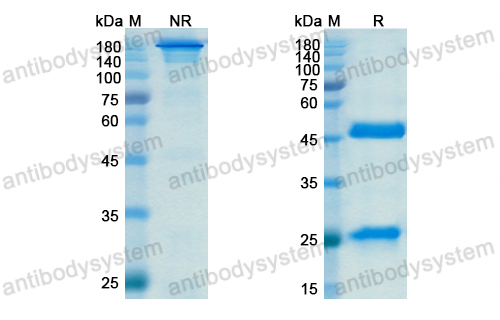
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
O95760
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
REGN-3500, SAR-440340, CAS: 2226742-52-3
Clone ID
Itepekimab
Topical delivery of a human single-domain antibody targeting IL-33 to inhibit mucosal inflammation., PMID:40500403
Mechanisms and Treatment of Type 2 High and Low Asthma Endotypes., PMID:40462371
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Biologic Therapies for Chronic Obstructive Pulmonary Disease: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials., PMID:39877958
Targeting alarmins in asthma: From bench to clinic., PMID:39855362
Role of Monoclonal Antibodies in the Management of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-analysis of Randomized Controlled Trials., PMID:39589286
Safety profile of drugs used in non-cystic fibrosis bronchiectasis: a narrative review., PMID:39372891
AERIFY-1/2: two phase 3, randomised, controlled trials of itepekimab in former smokers with moderate-to-severe COPD., PMID:39319046
Pharmacokinetics of Subcutaneous Itepekimab Injection With an Autoinjector Device and Prefilled Syringe in Healthy Participants., PMID:39308293
Correction to Pharmacokinetics and pharmacodynamics of itepekimab in adults with moderate-to-severe atopic dermatitis: Results from two terminated phase II trials., PMID:39300731
Pharmacokinetics and pharmacodynamics of itepekimab in adults with moderate-to-severe atopic dermatitis: Results from two terminated phase II trials., PMID:39077906
Association of Area Deprivation Index with Adherence to Proposed Regimen in Patients with Sarcoidosis in Detroit, Michigan., PMID:38940707
Towards precision medicine in COPD: Targeting type 2 cytokines and alarmins., PMID:38762432
[Not Available]., PMID:37316246
Mapping knowledge structure and research of the biologic treatment of asthma: A bibliometric study., PMID:36845128
Impact of Biologic Therapy on the Small Airways Asthma Phenotype., PMID:36239786
Eosinophilia Induced by Blocking the IL-4/IL-13 Pathway: Potential Mechanisms and Clinical Outcomes., PMID:35522053
Specific Therapy for T2 Asthma., PMID:35455709
Targeting Downstream Type 2 Cytokines or Upstream Epithelial Alarmins for Severe Asthma., PMID:35131510
Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma., PMID:34706171
Pharmacokinetics and pharmacodynamics of itepekimab in healthy adults and patients with asthma: Phase I first-in-human and first-in-patient trials., PMID:34523807
Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial., PMID:34302758