Monkeypox virus (MPXV), was first identified in 1958 when the virus was isolated from an infected Cynomolgus macaques at Denmark. However, it wasn’t until 1970 that the first human cases were documented in the Democratic Republic of the Congo (DRC). This occurred during intensified efforts to eradicate smallpox. The initial case involved a 9-month-old child who was taken to Basankusu Hospital with suspected smallpox, marking the beginning of human monkeypox (HMPX) as a recognized disease.
The Monkeypox virus (MPXV) appears as a brick- or ovoid-shaped enveloped virus, approximately 220-250 nm in size. Despite being a DNA virus, MPXV encodes all the necessary proteins for viral DNA replication, transcription, virion assembly, and egress, allowing it to remain in the cytoplasm of infected cells for its entire life cycle. Its genome, composed of 196,858 bp of double-stranded DNA, includes around 200 genes and two telomeres with identical but oppositely oriented sequences of short tandem repeats.
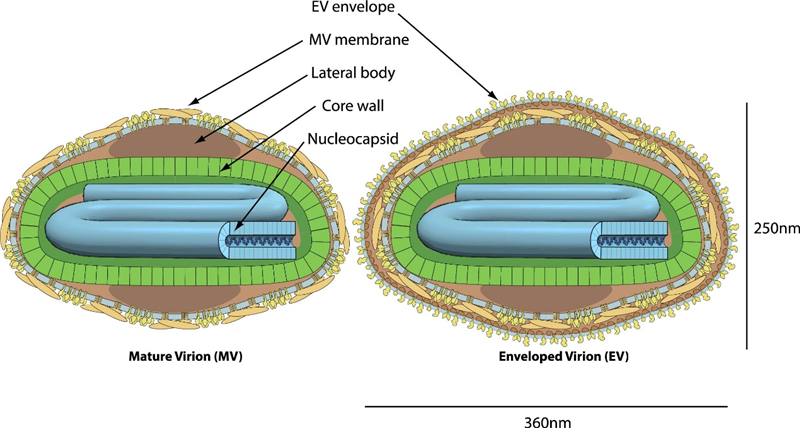
Fig. 1 Schematic representation of Monkeypox virus (viralzone)
Genomic studies have classified MPXV into two primary clades: the Central African/Congo Basin clade (Clade 1) and the West African clade (Clade 2). Clade 1, prevalent in Central Africa, is associated with higher fatality rates, ranging from 1% to 12%. Recently, a new sublineage within Clade 1, called Clade 1b, has been identified, which primarily infects children. Clade 2, found mainly in West Africa, is less virulent, with a fatality rate of less than 0.1%. In 2022, a new lineage closely related to Clade 2, known as Clade 2b, was identified during a global outbreak characterized by extensive human-to-human transmission.
MPXV primarily transmits from animals to humans. Bites or scratches from infected animals present a higher risk. Human-to-human transmission of MPXV can occur through respiratory secretions, direct contact, vertical transmission, percutaneous transmission, or indirect contact via fomites. Recently, this mode of transmission has been particularly prevalent among men who have sex with men.
The virus can be diagnosed through RT-PCR, targeting conserved regions of the extracellular envelope protein gene (B6R) and the DNA polymerase gene (E9L). JYNNEOS, a live virus vaccine, this non-replicating vaccine is specifically designed to protect against monkeypox. In terms of treatment, Tecovirimat is licensed for use in severe cases of monkeypox or for individuals at risk of developing serious disease.
A35R
A35R, a crucial component of EEV, plays a pivotal role in monkeypox virus transmission, serves as a vital target for vaccine development, and holds potential for serological detection. A35R is homologous to the VACV outer membrane-specific protein A33R, which is an envelope virus-specific protein expressed both early and late after infection and is essential for effective cell-to-cell transmission. The absence of A33R significantly reduces VACV infectivity, making it a promising target for antibody neutralization. Animal models have demonstrated that an A33R-based vaccine provides protective immunity and induces the production of neutralizing antibodies. Due to the limited information available on A35R, comparisons are often drawn from VACV A33R studies. However, A35R's significance in monkeypox virus transmission is well established, and mRNA vaccines targeting A35R have shown promising results.
E8L
E8L is a cell surface-binding protein that is suggested to bind to host cell surface chondroitin sulphate proteoglycans (CSPG). The binding of the E8L protein to a cluster of gangliosides GM1, which mimics a lipid raft domain, is driven by both shape and electrostatic surface potential complementarities. Three potential B cell epitopes, 43-62 (VRINFKGGYISGGFLPNEYV), 94-113 (VHWNKKKYSSYEEAKKHDDG), and 204-223 (SSSNHEGKPHYITENYRNPY), have been predicted as promising vaccine candidates.
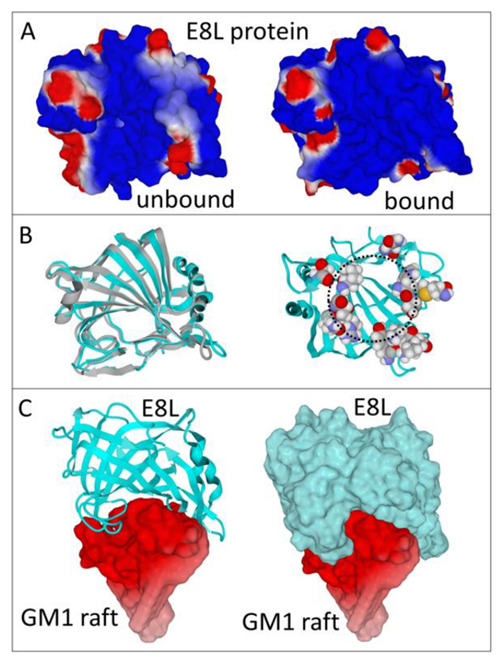
Fig. 2 Structure of E8L protein predicted by Robetta server (Fantini et al., 2022)
M1R
M1R is a highly conserved virion membrane protein (L1 ortholog), probably binds to host cell entry receptors. Form an entry fusion complex with other proteins. During cell infection, this complex mediates entry of the virion into the host cytoplasm.
A29L
The MPXV A29L protein, a vesicle membrane protein on the surface of IMV particles, is crucial for virion assembly and virus-host interactions. As a homologue of the VACV A27L protein, A29L aids in viral attachment to the host cell membrane and functions as both a transcription factor and an enzyme for viral replication. The A29L epitope (21-49 aa), located near the glycosaminoglycan (GAG) binding region, is a known target for neutralizing antibodies, interfering with viral adhesion to host cells.
B6R
The B6R gene encodes a 40 kDa type I transmembrane protein that functions as an extracellular envelope protein and is a specific target of MPXV. B6R is an ortholog of the VACV B5R gene, and a 35 kDa proteolytic fragment of B5R is also secreted from infected cells.
D14L
D14L is a component of the monkeypox inhibitor of complement enzymes (MOPICE). It protects the virus against complement attack by inhibiting both the classical and alternative pathways of complement activation, binding to C3b and C4b.
H3L
H3L binds to heparan sulfate on the cell surface, potentially facilitating virion attachment to target cells. H3L induces transcriptional perturbations and cellular injuries by upregulating IL1A expression.
Reference
1. Chen, S., Huang, G., and Liu, J. (2024). Monkeypox virus protein H3L induces injuries in human and mouse. Cell Death & Disease 15, 607.
2. Dighriri, I.M., Braiji, S.H., AlAnazi, M.M., Ayyashi, M.J., Khubrani, A.A., Khormi, Y.B., Shbeir, L.A., Alatif, S.I., and Alfagih, A.E. (2022). The Global Human Monkeypox Outbreak and Management: A Comprehensive Literature Review. Cureus 14, e32557.
3. Fantini, J., Chahinian, H., and Yahi, N. (2022). A Vaccine Strategy Based on the Identification of an Annular Ganglioside Binding Motif in Monkeypox Virus Protein E8L. Viruses 14, 2531.
4. Ghaseminia, M. (2023). Preventing monkeypox outbreaks: Focus on diagnosis, care, treatment, and vaccination. J Clin Transl Sci 7, e60.
5. Hatmal, M.M., Al-Hatamleh, M.A.I., Olaimat, A.N., Ahmad, S., Hasan, H., Ahmad Suhaimi, N.A., Albakri, K.A., Abedalbaset Alzyoud, A., Kadir, R., and Mohamud, R. (2022). Comprehensive literature review of monkeypox. Emerg Microbes Infect 11, 2600-2631.
6. Kang, Y., Yu, Y., and Xu, S. (2023). Human monkeypox infection threat: A comprehensive overview. PLoS Negl Trop Dis 17, e0011246.
7. Khani, E., Afsharirad, B., and Entezari-Maleki, T. (2023). Monkeypox treatment: Current evidence and future perspectives. J Med Virol 95, e28229.
8. Khattak, S., Rauf, M.A., Ali, Y., Yousaf, M.T., Liu, Z., Wu, D.D., and Ji, X.Y. (2022). The monkeypox diagnosis, treatments and prevention: A review. Front Cell Infect Microbiol 12, 1088471.
9. Li, M., Ren, Z., Wang, Y., Jiang, Y., Yang, M., Li, D., Chen, J., Liang, Z., Lin, Y., Zeng, Z., et al. (2023). Three neutralizing mAbs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus. Emerging Microbes & Infections 12, 2223669.
10. Masirika, L.M., Kumar, A., Dutt, M., Ostadgavahi, A.T., Hewins, B., Bubala, N.M., Steeven, B.K., Kumbana Mweshi, F., Mambo, L.M., and Mbiribindi, J.B. (2024). Complete genome sequencing, annotation, and mutational profiling of the novel Clade I human Mpox virus, Kamituga strain.
11. Meng, N., Cheng, X., Sun, M., Zhang, Y., Sun, X., Liu, X., and Chen, J. (2023). Screening, Expression and Identification of Nanobody Against Monkeypox Virus A35R. Int J Nanomedicine 18, 7173-7181.
12. Mitja, O., Ogoina, D., Titanji, B.K., Galvan, C., Muyembe, J.J., Marks, M., and Orkin, C.M. (2023). Monkeypox. Lancet 401, 60-74.
13. Nalca, A., Rimoin, A.W., Bavari, S., and Whitehouse, C.A. (2005). Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis 41, 1765-1771.
14. Parker, S., Nuara, A., Buller, R.M., and Schultz, D.A. (2007). Human monkeypox: an emerging zoonotic disease. Future Microbiol 2, 17-34.
15. Vakaniaki, E.H., Kacita, C., Kinganda-Lusamaki, E., O'Toole, A., Wawina-Bokalanga, T., Mukadi-Bamuleka, D., Amuri-Aziza, A., Malyamungu-Bubala, N., Mweshi-Kumbana, F., Mutimbwa-Mambo, L., et al. (2024). Sustained human outbreak of a new MPXV clade I lineage in eastern Democratic Republic of the Congo. Nat Med.
16. Zahmatyar, M., Fazlollahi, A., Motamedi, A., Zolfi, M., Seyedi, F., Nejadghaderi, S.A., Sullman, M.J.M., Mohammadinasab, R., Kolahi, A.A., Arshi, S., et al. (2023). Human monkeypox: history, presentations, transmission, epidemiology, diagnosis, treatment, and prevention. Front Med (Lausanne) 10, 1157670.
Host species: Human
Isotype: IgG1, kappa
Applications: ELISA, Neutralization, WB
Accession: NP_536566.1
Host species: Human
Isotype: IgA, kappa
Applications: ELISA, IB, Neutralization, WB
Accession: NP_536566.1
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: P20499
Protein length: Met1-Val269
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: AAL40631.1
Protein length: Met1-Asn344
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: E. coli
Accession: AAU01375.1
Protein length: Met1-Thr300
Applications: ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Expression system: Mammalian cells
Accession: Q8V502
Protein length: Ala3-Gly183
Host species: Human
Isotype: IgG1
Applications: ELISA
Accession: NP_536594.1