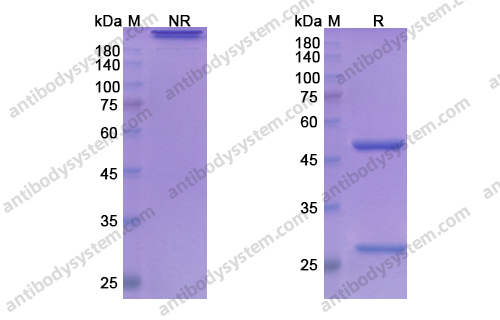
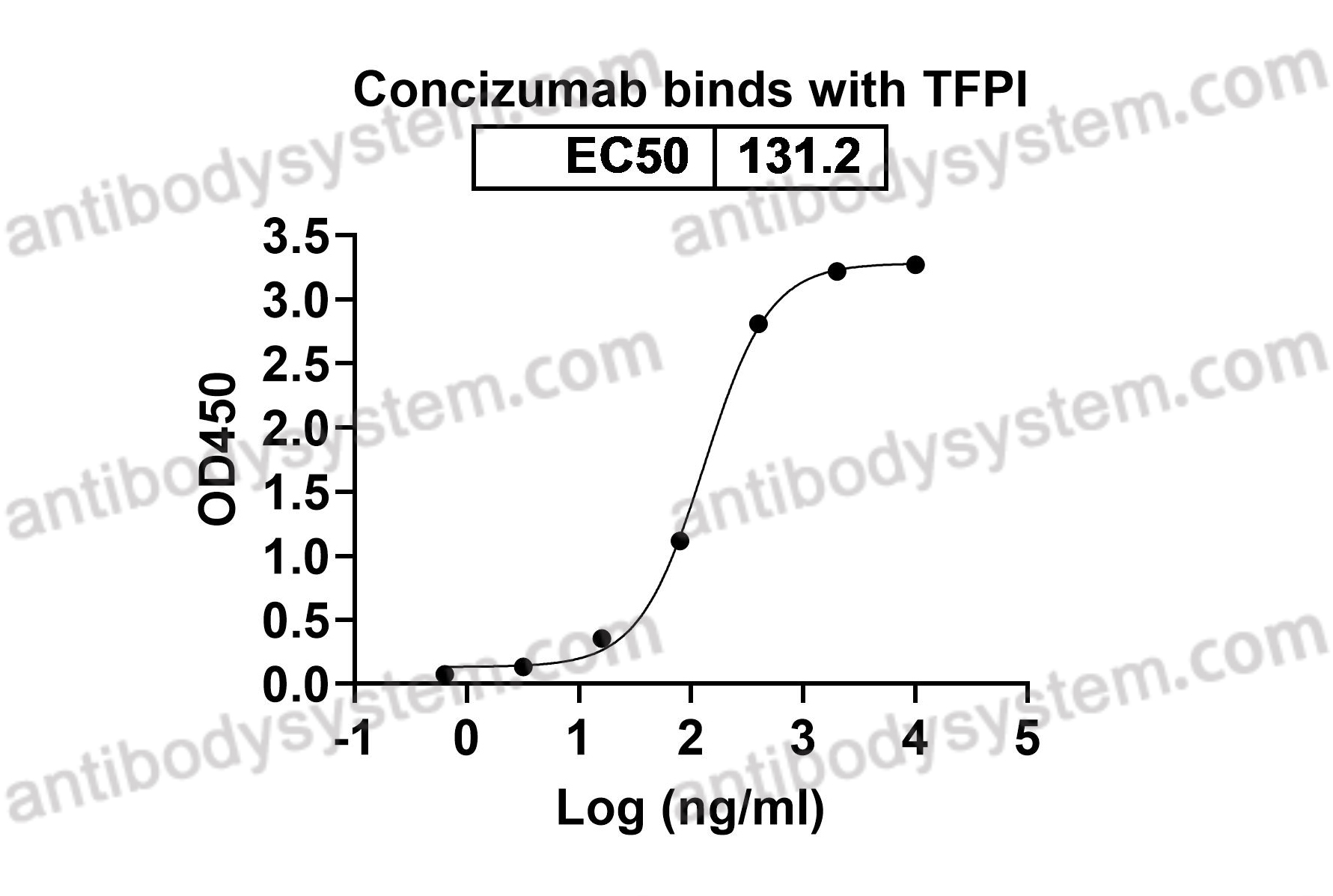
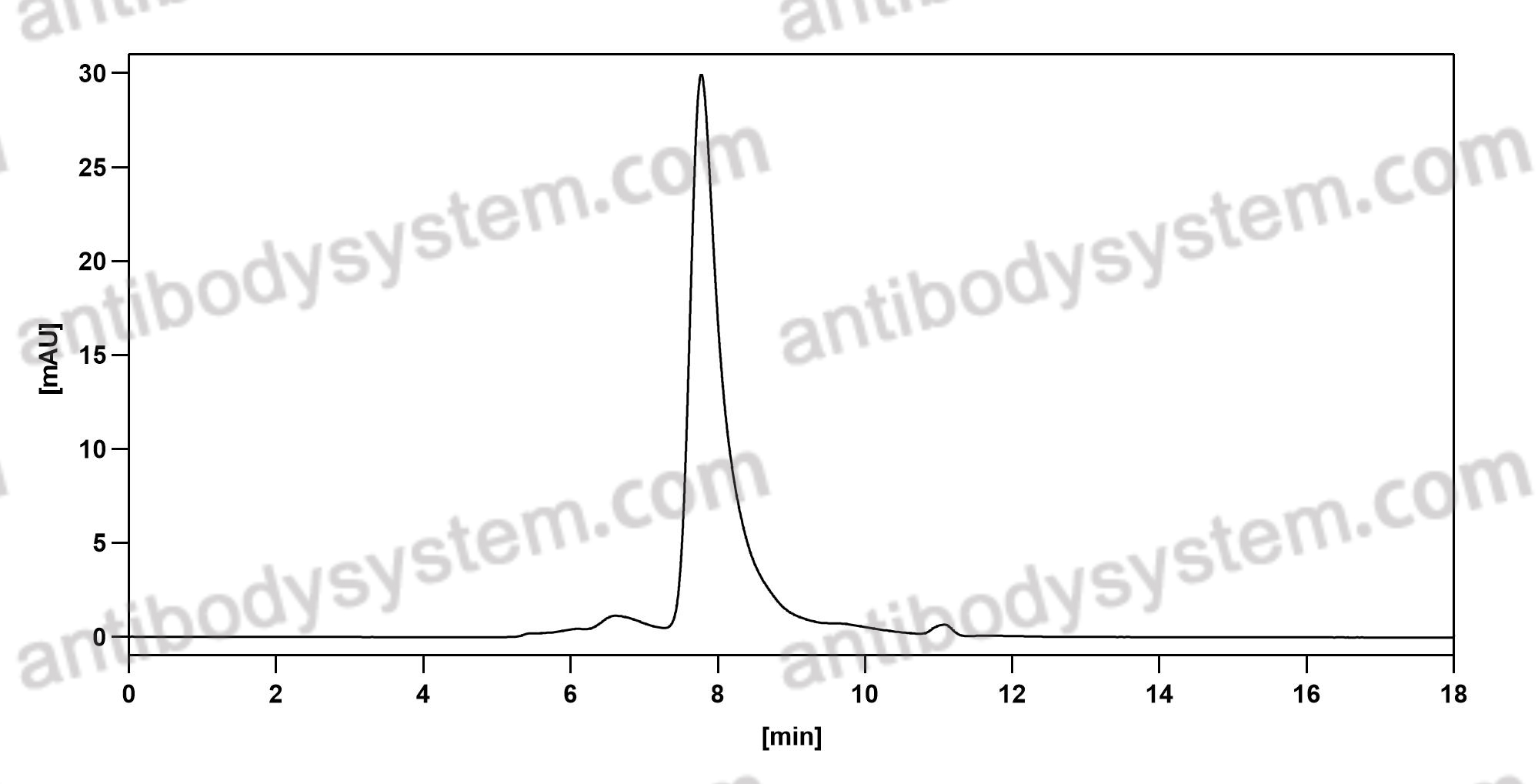
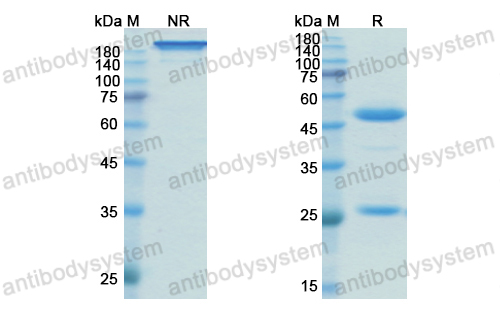
Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results, PMID: 31444162
Inhibition of Tissue Factor Pathway Inhibitor (TFPI) as a Treatment for Haemophilia: Rationale with Focus on Concizumab, PMID: 29845491
Concizumab promotes haemostasis via a tissue factor-factor VIIa-dependent mechanism supporting prophylactic treatment of haemophilia: Results from a rabbit haemophilia bleeding model, PMID: 31609513
Administration of recombinant FVIIa (rFVIIa) to concizumab-dosed monkeys is safe, and concizumab does not affect the potency of rFVIIa in hemophilic rabbits, PMID: 30614620
Concizumab restores thrombin generation potential in patients with haemophilia: Pharmacokinetic/pharmacodynamic modelling results of concizumab phase 1/1b data, PMID: 30408848
A randomized trial of safety, pharmacokinetics and pharmacodynamics of concizumab in people with hemophilia A, PMID: 30137664
Concizumab, an anti-tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay, PMID: 28594458
A systems pharmacokinetic/pharmacodynamic model for concizumab to explore the potential of anti-TFPI recycling antibodies, PMID: 31394258
The availability of new drugs for hemophilia treatment, PMID: 32515633
Concizumab: a novel anti-TFPI therapeutic for hemophilia, PMID: 33570646
Paradigm shift for the treatment of hereditary haemophilia: Towards precision medicine, PMID: 31676141
Non-factor replacement therapy for haemophilia: a current update, PMID: 29517971
Safety and pharmacokinetics of anti-TFPI antibody (concizumab) in healthy volunteers and patients with hemophilia: a randomized first human dose trial, PMID: 25641556
Pharmacokinetics of an anti-TFPI monoclonal antibody (concizumab) blocking the TFPI interaction with the active site of FXa in Cynomolgus monkeys after iv and sc administration, PMID: 24568891
Thrombin generation potential in the presence of concizumab and rFVIIa, APCC, rFVIII, or rFIX: In vitro and ex vivo analyses, PMID: 33819375
Correction to: Inhibition of Tissue Factor Pathway Inhibitor (TFPI) as a Treatment for Haemophilia: Rationale with Focus on Concizumab, PMID: 29931593
Investigational drugs to treat hemophilia, PMID: 32008381
Hemophilia in a Changing Treatment Landscape, PMID: 31030810
Balancing act, PMID: 25597120
Target-mediated clearance and bio-distribution of a monoclonal antibody against the Kunitz-type protease inhibitor 2 domain of Tissue Factor Pathway Inhibitor, PMID: 24393663
Prophylactic treatment in hemophilic patients with inhibitors, PMID: 31517711
Advances in the Treatment of Hemophilia: Implications for Laboratory Testing, PMID: 30282700
Current and emerging biologics for the treatment of hemophilia, PMID: 31039049
New therapies using nonfactor products for patients with hemophilia and inhibitors, PMID: 30559263
TFPI blockade: removing coagulation's brakes, PMID: 31778542
Emerging genetic and pharmacologic therapies for controlling hemostasis: beyond recombinant clotting factors, PMID: 26637698
Emerging drugs for the treatment of hemophilia A and B, PMID: 27547884
Hemostatic properties of a TFPI antibody, PMID: 22405586
Light and shadows of the new therapies for haemophilia treatment in the COVID-19 era, PMID: 32955428
Attempting to remedy sub-optimal medication adherence in haemophilia: The rationale for repeated ultrasound visualisations of the patient's joint status, PMID: 30146094
Treatment and prevention of bleeding in congenital hemophilia A patients with inhibitors, PMID: 30093246
Alternative therapies for the management of inhibitors, PMID: 27405674
The Potential Close Future of Hemophilia Treatment - Gene Therapy, TFPI Inhibition, Antithrombin Silencing, and Mimicking Factor VIII with an Engineered Antibody, PMID: 29765291
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management, PMID: 34026796
Post-authorization pharmacovigilance for hemophilia in Europe and the USA: Independence and transparency are keys, PMID: 33810898
Understanding the evolution of coverage policies for prophylaxis treatments of hemophilia A without inhibitors: a payer Delphi panel, PMID: 33843253
Real-World Early Treatment with Room Temperature-Stable Recombinant Factor VIIa in Hemophilia A/B and Inhibitors: SMART-7™ Post Hoc Analyses, PMID: 31249918
Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model, PMID: 22563084
Anti-tissue factor pathway inhibitors for hemophilia: are these treatments the answer to overcoming current treatment limitations?, PMID:40515600
Evaluating the Safety and Efficacy of Concizumab in Hemophilia A/B Patients: A Systematic Review., PMID:40368339
Concizumab, a Non-Replacement Therapy for Persons with Hemophilia with Inhibitors., PMID:40363993
Case Report: Severe hemophilia B patient with inhibitor and anaphylaxis reaction to FIX, successfully managed with concizumab prophylaxis therapy., PMID:40356785
Concizumab (Alhemo) for hemophilia A and B with inhibitors., PMID:40324964
Revolutionizing Treatment Strategies through Inhibition of Tissue Factor Pathway Inhibitor: A Promising Therapeutic Approach for Hemophilia Management., PMID:40200623
Erratum: The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference [Corrigendum]., PMID:40171517
Concizumab prophylaxis in people with hemophilia A or B without inhibitors: patient-reported outcome results from the phase 3 explorer8 study., PMID:40166710
Advances in Development of Drug Treatment for Hemophilia with Inhibitors., PMID:39698264
Non-factor Therapies for Hemophilia: Achievements and Perspectives., PMID:39613145
Examining downstream effects of concizumab in hemophilia A with a mathematical modeling approach., PMID:39536817
Concizumab prophylaxis in people with haemophilia A or haemophilia B without inhibitors (explorer8): a prospective, multicentre, open-label, randomised, phase 3a trial., PMID:39521008
The Concizumab Pen-Injector is Easy to Use and Preferred by Hemophilia Patients and Caregivers: A Usability Study Assessing Pen-Injector Handling and Preference., PMID:39161804
Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: patient-reported outcome results from the phase 3 explorer7 study., PMID:39099801
Disease and treatment burden of patients with haemophilia entering the explorer6 non-interventional study., PMID:39030946
Minimal interference of concizumab with standard clinical coagulation laboratory assays - An in vitro study., PMID:38924198
Targeting tissue factor pathway inhibitor with concizumab to improve hemostasis in patients with Glanzmann thrombasthenia: an in vitro study., PMID:38880178
Concizumab improves clot formation in hemophilia A under flow., PMID:38815755
Benefits and risks of non-factor therapies: Redefining haemophilia treatment goals in the era of new technologies., PMID:38481077
Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B., PMID:38411279
[Angiology and hemostasis: what's new in 2023]., PMID:38231093
Antibodies to watch in 2024., PMID:38178784
Phase 3 Trial of Concizumab in Hemophilia with Inhibitors., PMID:37646676
Correction to: Concizumab: First Approval., PMID:37535220
Concizumab: First Approval., PMID:37341887
The Use of Bypassing Treatment Strategies in Hemophilia and Their Effect on Laboratory Testing., PMID:37146647
The Implication of New Developments in Hemophilia Treatment on Its Laboratory Evaluation., PMID:36381757
Non-factor therapies for bleeding disorders: A primer for the general haematologist., PMID:36051064
[Progress of Non-Factor Products in Hemophilia Treatment--Review]., PMID:35981403
The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V., PMID:35955419
Current and future therapies for haemophilia-Beyond factor replacement therapies., PMID:35869698
Managing invasive procedures in haemophilia patients with limited resources, extended half-life concentrates or non-replacement therapies in 2022., PMID:35521735
Concizumab as a Subcutaneous Prophylactic Treatment Option for Patients with Hemophilia A or B: A Review of the Evidence and Patient's Perspectives., PMID:35465188
Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors., PMID:35290453
Monitoring of new therapies for hemophilia., PMID:35088769
Thrombin generation for monitoring hemostatic therapy in hemophilia A: A narrative review., PMID:35034413
Thrombosis and novel hemophilia therapies: the fine line between clotting and bleeding., PMID:34581771
Advances in the management of haemophilia: emerging treatments and their mechanisms., PMID:34521404
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management., PMID:34026796
Understanding the evolution of coverage policies for prophylaxis treatments of hemophilia A without inhibitors: a payer Delphi panel., PMID:33843253
Thrombin generation potential in the presence of concizumab and rFVIIa, APCC, rFVIII, or rFIX: In vitro and ex vivo analyses., PMID:33819375
Post-authorization pharmacovigilance for hemophilia in Europe and the USA: Independence and transparency are keys., PMID:33810898
Concizumab: a novel anti-TFPI therapeutic for hemophilia., PMID:33570646
Light and shadows of the new therapies for haemophilia treatment in the COVID-19 era., PMID:32955428
The availability of new drugs for hemophilia treatment., PMID:32515633
Investigational drugs to treat hemophilia., PMID:32008381
TFPI blockade: removing coagulation's brakes., PMID:31778542
Paradigm shift for the treatment of hereditary haemophilia: Towards precision medicine., PMID:31676141
Concizumab promotes haemostasis via a tissue factor-factor VIIa-dependent mechanism supporting prophylactic treatment of haemophilia: Results from a rabbit haemophilia bleeding model., PMID:31609513