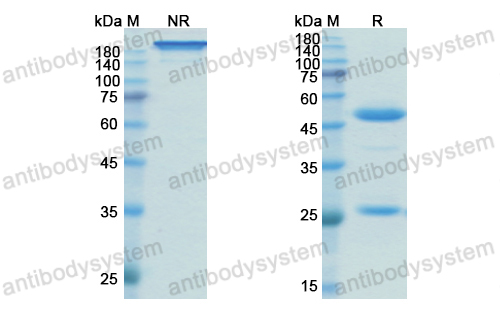
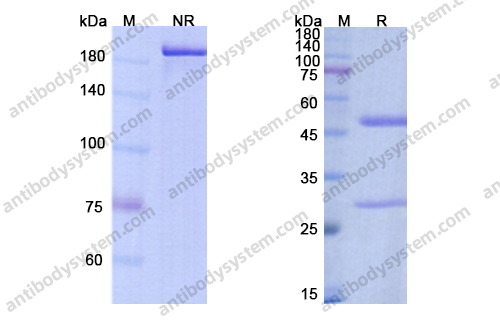
Catalog No.
DHB90008
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Chimeric
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
CPAMD4, C5, C3 and PZP-like alpha-2-macroglobulin domain-containing protein 4, Complement C5
Concentration
3.13 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01031
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CaCP-29, IFX-1, CAS: 2250440-41-4
Clone ID
Vilobelimab
Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial, PMID: 33015643
Complement inhibition in ANCA vasculitis, PMID: 31631015
New Therapeutic Targets in Antineutrophil Cytoplasm Antibody-Associated Vasculitis, PMID: 32562366
Pyoderma gangrenosum: proposed pathogenesis and current use of biologics with an emphasis on complement C5a inhibitor IFX-1, PMID: 32880206
Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: an open-label single-arm trial in patients not eligible for adalimumab, PMID: 31954061
Immunomodulatory therapies for SARS-CoV-2 infection: a systematic literature review to inform EULAR points to consider, PMID: 33589438
2025 Clinical Practice Guideline Update by the Infectious Diseases Society of America on the Treatment and Management of COVID-19: Vilobelimab., PMID:40402009
Therapeutics controversies in antineutrophilic cytoplasmic antibody-associated vasculitis., PMID:40392453
Regional comparison of efficacy and safety for vilobelimab in critically ill, invasively mechanically ventilated COVID-19 patients., PMID:40250846
Updates in innovation of the treatment of pyoderma gangrenosum., PMID:39720859
Limitation of site-stratified cox regression analysis in survival data: a cautionary tale of the PANAMO phase III randomized, controlled study in critically ill COVID-19 patients., PMID:39695734
An evaluation of vilobelimab (anti-C5a) as a cost-effective option to treat severely ill mechanically ventilated patients with COVID-19., PMID:39475087
Functional dependence following intensive care unit-treated sepsis: three-year follow-up results from the prospective Mid-German Sepsis Cohort (MSC)., PMID:39308983
Structure of Designer Antibody-like Peptides Binding to the Human C5a with Potential to Modulate the C5a Receptor Signaling., PMID:39051153
A review on the current approaches and perspectives of Covid-19 treatment., PMID:39007473
Heterogeneity of treatment effect of vilobelimab in COVID-19: a secondary analysis of a randomised controlled trial., PMID:38943192
Treatment of Pyoderma Gangrenosum With Vilobelimab., PMID:38922593
Comparative efficacy and therapeutic positioning of biologics in hidradenitis suppurativa: A systematic review with network meta-analysis of randomised trials., PMID:38595016
Choosing immunomodulating therapies for the treatment of COVID-19: recommendations based on placebo-controlled trial evidence., PMID:38182048
Race, Ethnicity, Sex, Gender, Socioeconomic Status, and Representativeness of Race and Ethnicity in ANCA Vasculitis Randomized Trials., PMID:38150245
Rational design of antibody-like peptides for targeting the human complement fragment protein C5a., PMID:37933678
Targeting C5a is beneficial in critically ill COVID-19 patients., PMID:37717470
Optimizing the use of vilobelimab for the treatment of COVID-19., PMID:37421632
Pharmacokinetic analysis of vilobelimab, anaphylatoxin C5a and antidrug antibodies in PANAMO: a phase 3 study in critically ill, invasively mechanically ventilated COVID-19 patients., PMID:37332066
COVID-19 update: An EUA for vilobelimab (Gohibic) for COVID-19., PMID:37216201
FDA Approves Vilobelimab for Emergency Use in Hospitalized Adults., PMID:37075230
Anti-C5a antibody vilobelimab treatment and the effect on biomarkers of inflammation and coagulation in patients with severe COVID-19: a substudy of the phase 2 PANAMO trial., PMID:36566174
Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial., PMID:36087611
Evaluation of Rituximab for Induction and Maintenance Therapy in Patients 75 Years and Older With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis., PMID:35802372
ANCA-Associated Vasculitic Neuropathies: A Review., PMID:35044596
The anti-C5a antibody vilobelimab efficiently inhibits C5a in patients with severe COVID-19., PMID:35029045
Efficacy and Safety of Vilobelimab (IFX-1), a Novel Monoclonal Anti-C5a Antibody, in Patients With Early Severe Sepsis or Septic Shock-A Randomized, Placebo-Controlled, Double-Blind, Multicenter, Phase IIa Trial (SCIENS Study)., PMID:34806021
Immunomodulatory therapies for SARS-CoV-2 infection: a systematic literature review to inform EULAR points to consider., PMID:33589438
Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial., PMID:33015643
Pyoderma gangrenosum: proposed pathogenesis and current use of biologics with an emphasis on complement C5a inhibitor IFX-1., PMID:32880206
New Therapeutic Targets in Antineutrophil Cytoplasm Antibody-Associated Vasculitis., PMID:32562366
Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: an open-label single-arm trial in patients not eligible for adalimumab., PMID:31954061
Complement inhibition in ANCA vasculitis., PMID:31631015