Catalog No.
DHH02219
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
Programmed cell death protein 1, Protein PD-1, hPD-1, PD1, PDCD1, CD279
Concentration
1.65 mg/ml
Endotoxin level
Please contact with the lab for this information.
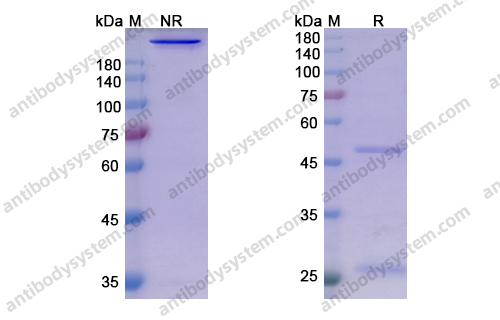
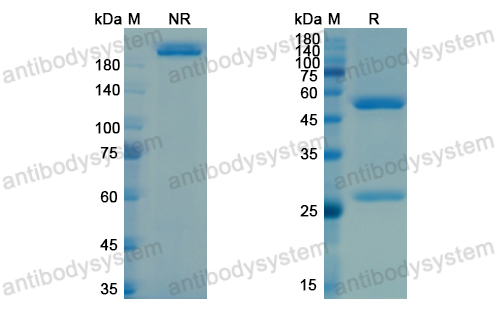
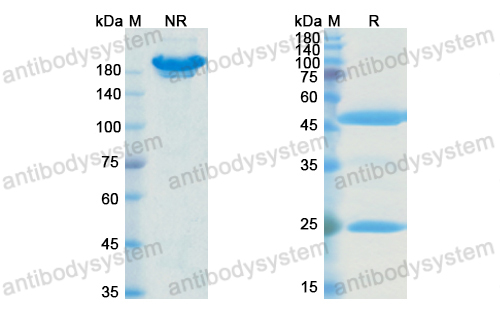
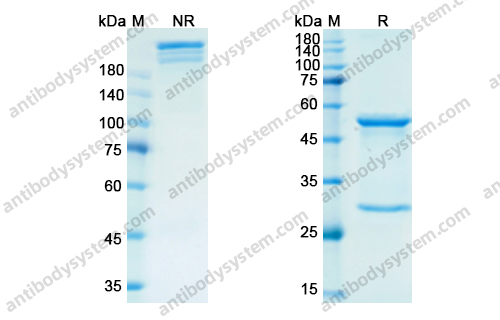
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q15116
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
PF-06801591, CAS: 2206792-50-7
Clone ID
Sasanlimab
Sasanlimab plus BCG in BCG-naive, high-risk non-muscle invasive bladder cancer: the randomized phase 3 CREST trial., PMID:40450141
Real-World and Clinical Trial Validation of a Deep Learning Radiomic Biomarker for PD-(L)1 Immune Checkpoint Inhibitor Response in Advanced Non-Small Cell Lung Cancer., PMID:39671539
Pharmacokinetics, safety, and efficacy of an alternative dosing regimen of sasanlimab in participants with advanced NSCLC and other malignancies., PMID:39281971
CREST: phase III study of sasanlimab and Bacillus Calmette-Guérin for patients with Bacillus Calmette-Guérin-naïve high-risk non-muscle-invasive bladder cancer., PMID:38189180
Analysis of phase III clinical trials in metastatic NSCLC to assess the correlation between QoL results and survival outcomes., PMID:37400832
A phase Ib/II dose expansion study of subcutaneous sasanlimab in patients with locally advanced or metastatic non-small-cell lung cancer and urothelial carcinoma., PMID:37385154
First-in-human, phase 1 study of PF-06753512, a vaccine-based immunotherapy regimen (VBIR), in non-metastatic hormone-sensitive biochemical recurrence and metastatic castration-resistant prostate cancer (mCRPC)., PMID:36948505
A phase II clinical trial of neoadjuvant sasanlimab and stereotactic body radiation therapy as an in situ vaccine for cisplatin-ineligible MIBC: the RAD VACCINE MIBC trial., PMID:35703113
Association of Tumor Mutational Burden and Immune Gene Expression with Response to PD-1 Blockade by Sasanlimab Across Tumor Types and Routes of Administration., PMID:34694529
Pharmacologic Properties and Preclinical Activity of Sasanlimab, A High-affinity Engineered Anti-Human PD-1 Antibody., PMID:32847983