Catalog No.
DHH02218
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
Programmed cell death protein 1, Protein PD-1, hPD-1, PD1, PDCD1, CD279
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
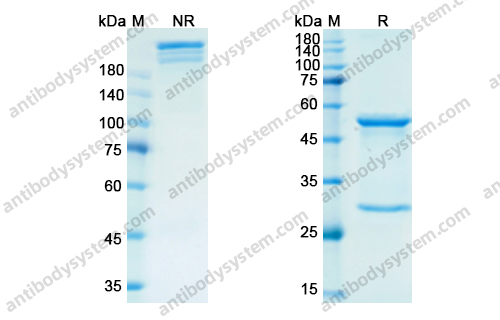
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q15116
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
INCMGA-00012, MGA-012, CAS: 2079108-44-2
Clone ID
Retifanlimab
MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma, PMID: 33263418
Emerging and Experimental Agents for Anal Cancer: What is New?, PMID: 33859504
Retifanlimab with carboplatin and paclitaxel for locally recurrent or metastatic squamous cell carcinoma of the anal canal (POD1UM-303/InterAACT-2): a global, phase 3 randomised controlled trial., PMID:40517007
Emerging advances and future opportunities in the molecular and therapeutic landscape of anal cancer., PMID:40360682
High-resolution crystal structure of PD-1 in complex with retifanlimab, the FDA-approved immune checkpoint blocking antibody for treating Merkel cell carcinoma., PMID:39632294
Updates in the Management of Advanced Nonmelanoma Skin Cancer., PMID:39244290
[Retifanlimab in metastatic Merkel cell carcinoma in first line]., PMID:39227198
Open-label, dose-escalation FIGHT-101 study of pemigatinib combined with targeted therapy, chemotherapy, or immunotherapy in patients with advanced malignancies., PMID:38986210
Secondary adrenocortical insufficiency after treatment with retifanlimab: a case report., PMID:38915406
Antitumor activity and safety of the PD-1 inhibitor retifanlimab in patients with recurrent microsatellite instability-high or deficient mismatch repair endometrial cancer: Final safety and efficacy results from cohort H of the POD1UM-101 phase I study., PMID:38824752
Retifanlimab in Advanced Penile Squamous Cell Carcinoma: The Phase 2 ORPHEUS Study., PMID:38749903
PD-1 inhibition with retifanlimab and/or arginase inhibition with INCB001158 in Japanese patients with solid tumors: A phase I study., PMID:38651187
Merkel Cell Carcinoma: Integrating Epidemiology, Immunology, and Therapeutic Updates., PMID:38649621
Atezolizumab plus modified docetaxel, cisplatin, and fluorouracil as first-line treatment for advanced anal cancer (SCARCE C17-02 PRODIGE 60): a randomised, non-comparative, phase 2 study., PMID:38547895
A phase II study of retifanlimab, a humanized anti-PD-1 monoclonal antibody, in patients with solid tumors (POD1UM-203)., PMID:38401247
A first-in-human phase I study of the PD-1 inhibitor, retifanlimab (INCMGA00012), in patients with advanced solid tumors (POD1UM-101)., PMID:38387109
Antibodies to watch in 2024., PMID:38178784
Retifanlimab-dlwr., PMID:37257157
Retifanlimab: First Approval., PMID:37184754
In brief: Retifanlimab (Zynyz) for Merkel cell carcinoma., PMID:37039620
Cutaneous, oral and genital lichenoid reactions associated with retifanlimab, a new PD-1 inhibitor., PMID:36433782
POD1UM-303/InterAACT 2: A phase III, global, randomized, double-blind study of retifanlimab or placebo plus carboplatin-paclitaxel in patients with locally advanced or metastatic squamous cell anal carcinoma., PMID:36091159
Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A., PMID:36029651
A phase II study of retifanlimab (INCMGA00012) in patients with squamous carcinoma of the anal canal who have progressed following platinum-based chemotherapy (POD1UM-202)., PMID:35816951
Emerging and Experimental Agents for Anal Cancer: What is New?, PMID:33859504
MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma., PMID:33263418