Catalog No.
VVV09601
Species reactivity
Chikungunya virus (strain S27-African prototype) (CHIKV), Chikungunya virus (CHIKV), Chikungunya virus (strain 37997) (CHIKV)
Host species
Human
Isotype
IgG1
Clonality
Monoclonal
Target
Structural polyprotein, p130, Capsid protein, 3.4.21.90, Coat protein, C, Precursor of protein E3/E2, p62, pE2, Assembly protein E3, Spike glycoprotein E2, E2 envelope glycoprotein, 6K protein, Spike glycoprotein E1, E1 envelope glycoprotein
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
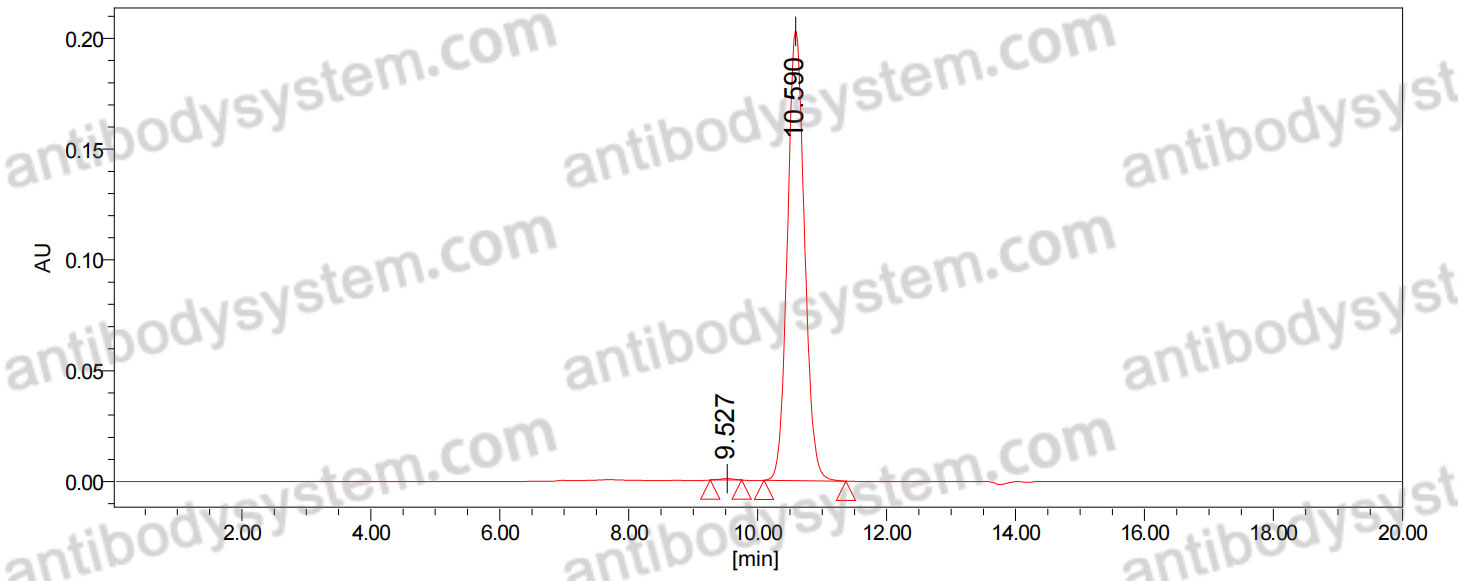
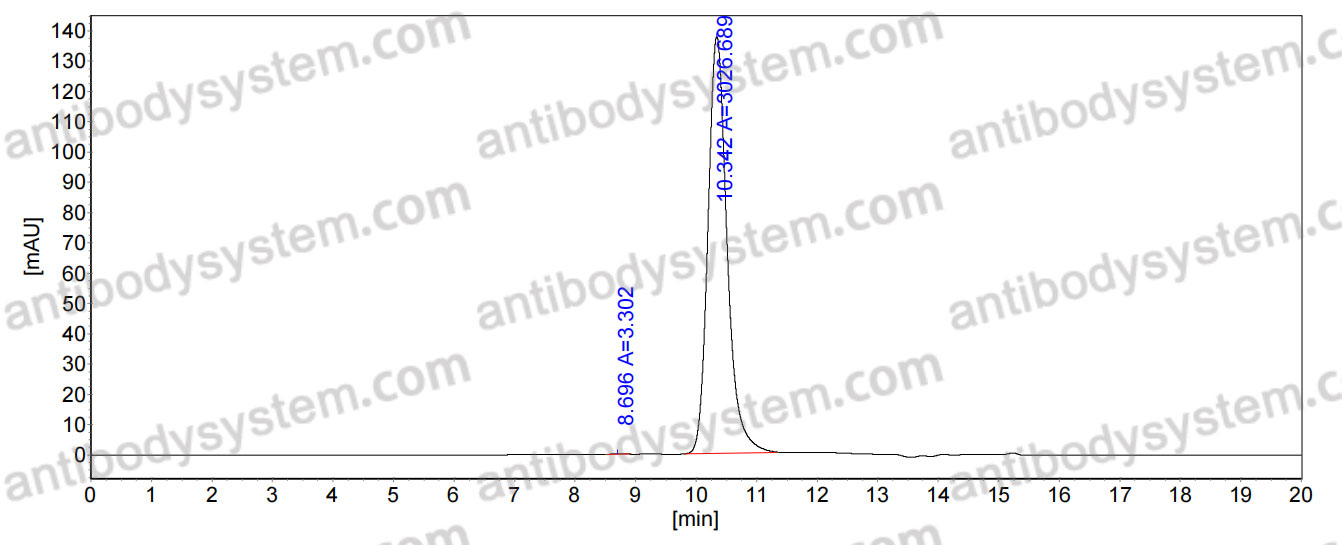
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q8JUX5, Q1H8W5, Q5XXP3
Applications
ELISA, Neutralization
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
m242
Seroprevalence of Chikungunya and O'nyong-nyong Viruses in Senegal, West Africa., PMID:40028687
Safety and immunogenicity of an adjuvanted chikungunya virus virus-like particle (CHIKV VLP) vaccine in previous recipients of other alphavirus vaccines versus alphavirus vaccine-naive controls: an open-label, parallel-group, age-matched, sex-matched, phase 2 randomised controlled study., PMID:39954701
Chikungunya virus virus-like particle vaccine is well tolerated and immunogenic in chikungunya seropositive individuals., PMID:37690874
Vaccine elicitation and structural basis for antibody protection against alphaviruses., PMID:37295404
Antibody seropositivity and endemicity of chikungunya and Zika viruses in Nigeria., PMID:36968287
Comparative Efficacy of Mayaro Virus-Like Particle Vaccines Produced in Insect or Mammalian Cells., PMID:36883812
Antigenicity and immunogenicity of chikungunya virus-like particles from mosquito cells., PMID:36434113
Safety and immunogenicity of PXVX0317, an aluminium hydroxide-adjuvanted chikungunya virus-like particle vaccine: a randomised, double-blind, parallel-group, phase 2 trial., PMID:35709798
Immunological implications of diverse production approaches for Chikungunya virus-like particle vaccines., PMID:35459557
Co-Immunization With CHIKV VLP and DNA Vaccines Induces a Promising Humoral Response in Mice., PMID:33868299
Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria., PMID:33803622
Effect of a Chikungunya Virus-Like Particle Vaccine on Safety and Tolerability Outcomes: A Randomized Clinical Trial., PMID:32286643
Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus., PMID:32132648
Bacteriophage Qβ virus-like particles displaying Chikungunya virus B-cell epitopes elicit high-titer E2 protein antibodies but fail to neutralize a Thailand strain of Chikungunya virus., PMID:32044164
The use of green fluorescent protein-tagged virus-like particles as a tracer in the early phase of chikungunya infection., PMID:31445103
Vaccination with a chikungunya virus-like particle vaccine exacerbates disease in aged mice., PMID:31026260
Development of a bead-based immunoassay using virus-like particles for detection of alphaviral humoral response., PMID:30998959
Whole-Inactivated and Virus-Like Particle Vaccine Strategies for Chikungunya Virus., PMID:27920180
Structural Studies of Chikungunya Virus-Like Particles Complexed with Human Antibodies: Neutralization and Cell-to-Cell Transmission., PMID:26537684
Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity., PMID:26504196
Do we need a vaccine against chikungunya?, PMID:25971340
[[Virus-like particle-based immunoglobulin M capture enzyme-linked immunosorbent assay for the detection of IgM antibodies against Chikungunya virus]., PMID:25868272
Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial., PMID:25132507
Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits., PMID:24099875
Structural analyses at pseudo atomic resolution of Chikungunya virus and antibodies show mechanisms of neutralization., PMID:23577234
A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection., PMID:20111039