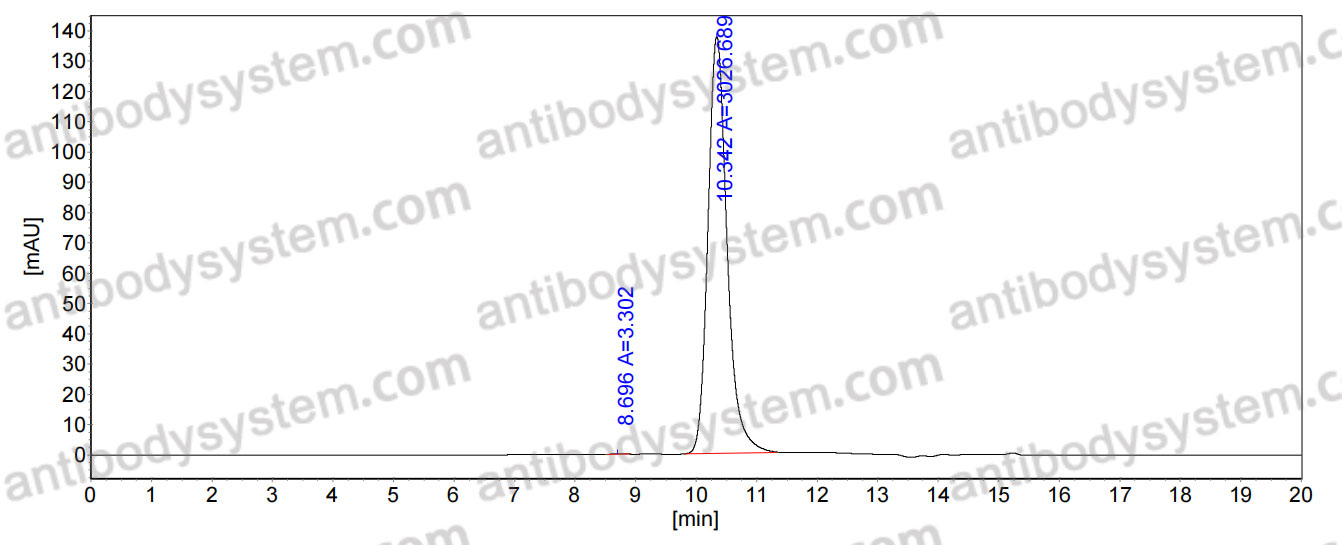
Structural basis of human cytomegalovirus neutralization by gB AD-5-specific potent antibodies., PMID:40382771
A human cytomegalovirus prefusion-like glycoprotein B subunit vaccine elicits humoral immunity similar to that of postfusion gB in mice., PMID:40338082
Human cytomegalovirus gH/gL/gO binding to PDGFRα provides a regulatory signal activating the fusion protein gB that can be blocked by neutralizing antibodies., PMID:40202318
Human cytomegalovirus gH/gL/gO binding to PDGFRα provides a regulatory signal activating the fusion protein gB that can be blocked by neutralizing antibodies., PMID:39829861
SPA14 liposomes combining saponin with fully synthetic TLR4 agonist provide adjuvanticity to hCMV vaccine candidate., PMID:39702373
Human cytomegalovirus: pathogenesis, prevention, and treatment., PMID:39585514
Structure-based design of a soluble human cytomegalovirus glycoprotein B antigen stabilized in a prefusion-like conformation., PMID:39231203
Human cytomegalovirus glycoprotein variants governing viral tropism and syncytium formation in epithelial cells and macrophages., PMID:38837351
Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine., PMID:38324766
Fully Human Herpesvirus-Specific Neutralizing IgG Antibodies Generated by EBV Immortalization of Splenocytes-Derived from Immunized Humanized Mice., PMID:38201224
Re-Evaluating Human Cytomegalovirus Vaccine Design: Prediction of T Cell Epitopes., PMID:38005961
Vaccination with a replication-defective cytomegalovirus vaccine elicits a glycoprotein B-specific monoclonal antibody repertoire distinct from natural infection., PMID:37816743
A temperature-dependent virus-binding assay reveals the presence of neutralizing antibodies in human cytomegalovirus gB vaccine recipients' sera., PMID:37310000
The cytomegalovirus gB/MF59 vaccine candidate induces antibodies against an antigenic domain controlling cell-to-cell spread., PMID:36823200
CRM197-conjugated multi antigen dominant epitope for effective human cytomegalovirus vaccine development., PMID:36252620
Endothelial Cell Infection by Guinea Pig Cytomegalovirus Is a Lytic or Persistent Infection Depending on Tissue Origin but Requires Viral Pentamer Complex and pp65 Tegument Protein., PMID:36000848
Neutralizing Antibodies to Human Cytomegalovirus Recombinant Proteins Reduce Infection in an Ex Vivo Model of Developing Human Placentas., PMID:35891239
Identification of functionally important domains of human cytomegalovirus gO that act after trimer binding to receptors., PMID:35452493
Horizontal Transmission of Cytomegalovirus in a Rhesus Model Despite High-Level, Vaccine-Elicited Neutralizing Antibody and T-Cell Responses., PMID:35413121
Neutralizing Antibodies Limit Cell-Associated Spread of Human Cytomegalovirus in Epithelial Cells and Fibroblasts., PMID:35215877
Dominant Antiviral CD8+ T Cell Responses Empower Prophylactic Antibody-Eliciting Vaccines Against Cytomegalovirus., PMID:35154089
Pre-existing immunity to cytomegalovirus in macaques influences human CMV vaccine responses in preclinical models., PMID:34393017
Characterization of gH/gL/pUL128-131 pentameric complex, gH/gL/gO trimeric complex, gB and gM/gN glycoproteins in a human cytomegalovirus using automated capillary western blots., PMID:34229890
A Novel Strain-Specific Neutralizing Epitope on Glycoprotein H of Human Cytomegalovirus., PMID:34160252
Mutagenesis of Human Cytomegalovirus Glycoprotein L Disproportionately Disrupts gH/gL/gO over gH/gL/pUL128-131., PMID:34132577
A conditionally replication-defective cytomegalovirus vaccine elicits potent and diverse functional monoclonal antibodies in a phase I clinical trial., PMID:34078915
A trimeric capable gB CMV vaccine provides limited protection against a highly cell associated and epithelial tropic strain of cytomegalovirus in guinea pigs., PMID:33729125
Rationally designed Human Cytomegalovirus gB nanoparticle vaccine with improved immunogenicity., PMID:33370407
Potent Bispecific Neutralizing Antibody Targeting Glycoprotein B and the gH/gL/pUL128/130/131 Complex of Human Cytomegalovirus., PMID:33361306
IgA binds to the AD-2 epitope of glycoprotein B and neutralizes human cytomegalovirus., PMID:33283275
Specificity and effector functions of non-neutralizing gB-specific monoclonal antibodies isolated from healthy individuals with human cytomegalovirus infection., PMID:32838941
Association of human cytomegalovirus (HCMV) neutralizing antibodies with antibodies to the HCMV glycoprotein complexes., PMID:32746933
Recognition of a highly conserved glycoprotein B epitope by a bivalent antibody neutralizing HCMV at a post-attachment step., PMID:32745149
Repertoire characterization and validation of gB-specific human IgGs directly cloned from humanized mice vaccinated with dendritic cells and protected against HCMV., PMID:32667948
Cell Fusion Induced by a Fusion-Active Form of Human Cytomegalovirus Glycoprotein B (gB) Is Inhibited by Antibodies Directed at Antigenic Domain 5 in the Ectodomain of gB., PMID:32641474
Human Cytomegalovirus Congenital (cCMV) Infection Following Primary and Nonprimary Maternal Infection: Perspectives of Prevention through Vaccine Development., PMID:32340180
Immunization with Human Cytomegalovirus Core Fusion Machinery and Accessory Envelope Proteins Elicit Strong Synergistic Neutralizing Activities., PMID:32294946
Adult and Cord Blood-Derived High-Affinity gB-CAR-T Cells Effectively React Against Human Cytomegalovirus Infections., PMID:32159399
Requirements for guinea pig cytomegalovirus tropism and antibody neutralization on placental amniotic sac cells., PMID:32068527
Human Cytomegalovirus Glycoprotein B Nucleoside-Modified mRNA Vaccine Elicits Antibody Responses with Greater Durability and Breadth than MF59-Adjuvanted gB Protein Immunization., PMID:32051265
Seronegative patients vaccinated with cytomegalovirus gB-MF59 vaccine have evidence of neutralising antibody responses against gB early post-transplantation., PMID:31735553
Neutralizing Monoclonal Antibodies Reduce Human Cytomegalovirus Infection and Spread in Developing Placentas., PMID:31569508
Immunization of Rabbits with Recombinant Human Cytomegalovirus Trimeric versus Monomeric gH/gL Protein Elicits Markedly Higher Titers of Antibody and Neutralization Activity., PMID:31261659
Neutralization of rhesus cytomegalovirus IL-10 reduces horizontal transmission and alters long-term immunity., PMID:31189602
Human cytomegalovirus (HCMV) infection/re-infection: development of a protective HCMV vaccine., PMID:30671581
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism., PMID:30544948
A Native Human Monoclonal Antibody Targeting HCMV gB (AD-2 Site I)., PMID:30544903
Comparison of vaccination with rhesus CMV (RhCMV) soluble gB with a RhCMV replication-defective virus deleted for MHC class I immune evasion genes in a RhCMV challenge model., PMID:30522906
Intrahost Dynamics of Human Cytomegalovirus Variants Acquired by Seronegative Glycoprotein B Vaccinees., PMID:30518646
Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response., PMID:30082162