Dengue virus (DENV) is one of the most important arboviruses and is known to cause dengue infection, which is mainly transmitted by Aedes aegypti.
According to WHO, DF is now a common disease in more than a 100 countries, including parts of Africa, the Western Pacific, the Americas, Southeast Asia, and the Eastern Mediterranean. The areas having the greatest influence include Southeast Asia, the Americas, and the Western Pacific, with Asia accounting for more than 70% of global disease.
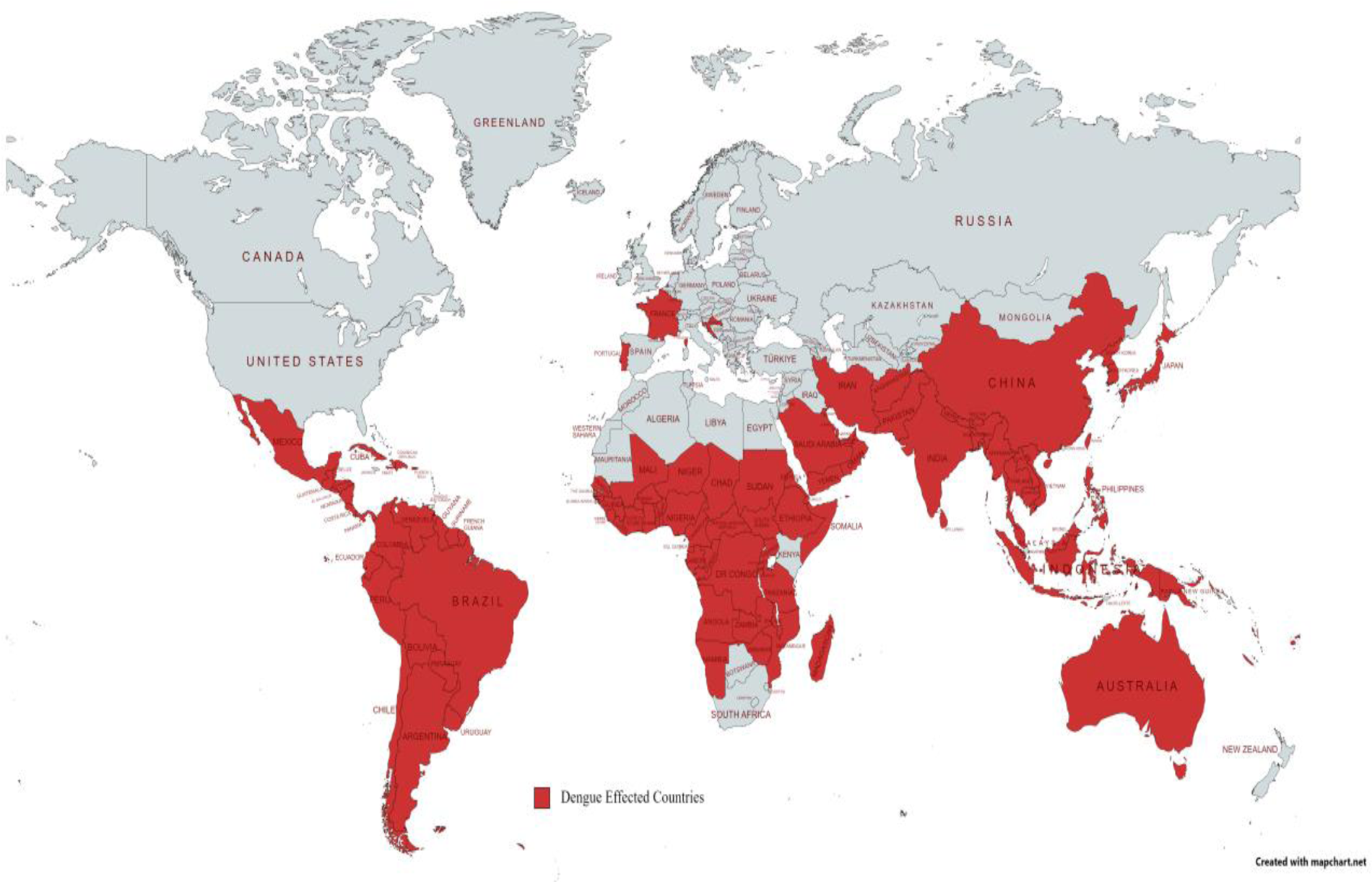
The worldwide distribution of dengue fever.
(J Water Health. 2023 Nov;21(11):1632-1650. )
Virus Structure
DENV belongs to the genus Flavivirus in the family Flaviviridae. The virus particles are spherical with a diameter of 45-55 nm, and there are four serotypes (DENV-1, DENV-2, DENV-3, and DENV-4), all of which can lead to human infections and cause severe illnesses, with DENV-4 being less transmissible and less widespread.
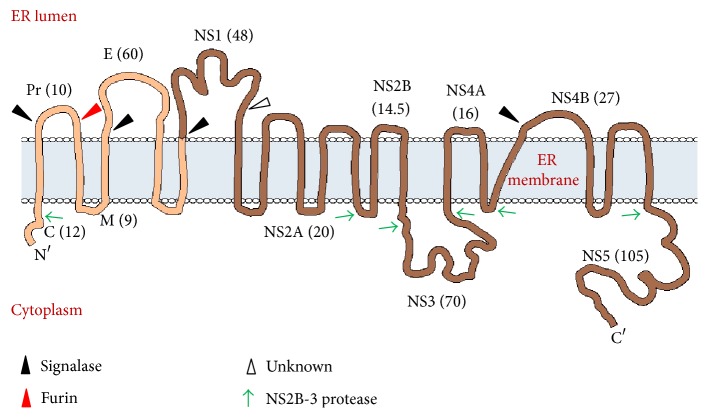
Genome organization and membrane topology of dengue virus.
(J Immunol Res. 2016:2016:6803098. )
Functions of DENV proteins
This RNA is translated into a single polyprotein which encodes for three structural proteins, namely, capsid (C), premembrane (prM), envelope (E), and 7 nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5).
Table 1. Functions of DENV proteins
|
Protein |
Function |
|
|
Structural |
Capsid (C) |
Binds and stabilizes viral RNA |
|
Premembrane/ membrane(prM/M) |
(i) Pr peptide functions as cap that protects the fusion peptide on E, thus preventing premature fusion |
|
|
Envelope (E) |
(i) Recognition and binding to the host cell |
|
|
Nonstructural (NS) |
NS1 |
(i) Viral RNA replication |
|
(ii) Viral defense through inhibition of complement activation |
||
|
NS2A |
Viral replication and assembly |
|
|
NS2B |
NS3 protease cofactor |
|
|
NS3 |
(i) Serine protease-cleaves viral polyprotein |
|
|
(ii) RNA helicase and RTPase/NTPase-viral RNA replication |
||
|
NS4A |
Induces membrane alterations and autophagy to enhance virus replication |
|
|
NS4B |
(i) Interacts with NS3-viral replication |
|
|
(ii) Blocks IFN-α/β-induced signal transduction and helps virus to escape host's innate immune response |
||
|
NS5 |
(i) Methyl transferase domain |
|
|
(ii) RNA-dependent RNA polymerase |
||
Pathogenesis of DF
DENV invades the human body through the bite of Aedes aegypti mosquito, enters the blood circulation after proliferation in the monocyte-macrophage system, forming the first viremia, then localizes in the reticuloendothelial system and lymphatic tissues, and replicates in peripheral blood mononuclear cells, macrophages in the tissues, and Kupffer cells in the liver, and enters the blood circulation again, forming the second viremia, and causing clinical symptoms. DENV combines with specific antibodies produced by the organism to form immune complexes, activating the complement system, leading to vasodilatation, congestion, increased permeability, extravasation of plasma proteins and blood components, causing pathophysiological changes such as hemoconcentration, hemorrhage and shock. At the same time, the virus can inhibit leukocyte and platelet production in the bone marrow. The bleeding mechanism may be related to thrombocytopenia and its dysfunction, coagulation factor depletion.
The pathogenesis of severe DF has not been fully elucidated so far, and factors such as antibody-dependent enhancement (ADE), cytokine storm, and viral virulence variation due to DENV secondary infection play an important role.
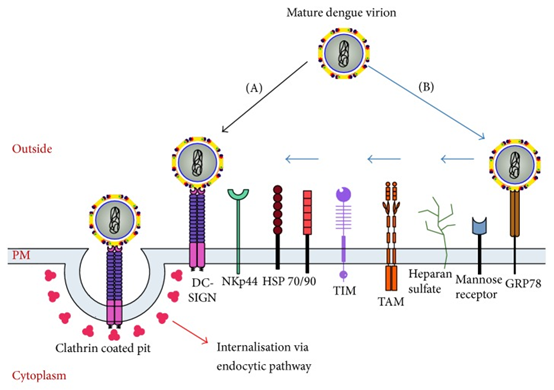
Schematic representation of the dengue virus entry process.
(J Immunol Res. 2016 Jul 20;2016:6803098. )
Development of Dengue Vaccine
Control of vectors and prevention of mosquito bites are important measures to prevent and control dengue virus infection. Secondly, dengue vaccination can also prevent DENV infection, but there is no safe and effective dengue virus vaccine available.
Dengue vaccine development has been a complex and challenging process due to the unique properties of the DENV and the need to provide protection against all four serotypes. In the early stages of dengue vaccine development, whole inactivated or live attenuated viruses were used. However, these early candidates faced challenges regarding safety, immunogenicity, and the ability to elicit a well-balanced immune response against each of the four serotypes. All four DENV serotypes (DENV 1–4) can cause DF, DHF, and DSS. An exclusive feature of DF is that an initial infection (primary infection) with any DENV serotype can provide homotypic and enduring protection against subsequent infections with that serotype alone. Subsequent secondary infections with another DENV serotype can result in fatal forms of DSS and DHF. It is widely accepted that heterologous antibodies bind to DENV and, via its Fcγ receptor, increase uptake into monocyte-lineage cells, increasing viral load and causing severe dengue. It is therefore essential that the DENV vaccine prevent infection by all four serotypes. Most dengue vaccines target either the viral structural protein NS1 or the envelope proteins present on the virus surface, yet there are still many safety concerns that must be addressed. In addition to the NS1 protein, other non-structural proteins such as NS2A, NS2B, NS3, NS4A, NS4B, and NS5 also present avenues for inhibitor development.
Despite the existing challenges for an ideal dengue vaccine, development of dengue vaccine candidates has progressed over the last decade and some of these have entered clinical trials in both endemic and nonendemic areas. Presently, two live attenuated dengue vaccines named as Dengvaxia® and Qdenga® have been approved for use in certain populations in dengue-endemic areas.
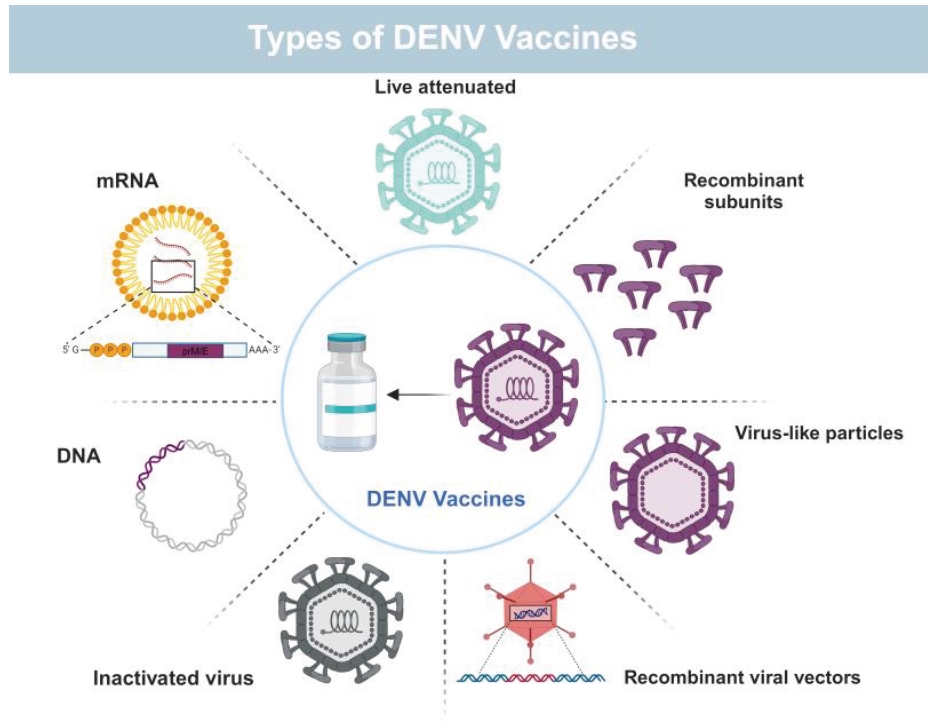
Types of DENV Vaccines
(J Microbiol. 2025 Feb;63(2):e2410018. )
Product List
|
Catalog |
Product Name |
|
Anti-DENV-2 E-DII/Envelope Protein E Antibody (2A10) |
|
|
Anti-Dengue Virus NS1 antibody |
|
|
InVivoMAb Anti-DENV2 Protein prM/prM Antibody (2H2) |
|
|
Recombinant DENV-2 NS1/Non-structural protein 1 Protein, N-His |
|
|
Anti-DENV-2 E-DII/Envelope Protein E Antibody (2A10) |
|
|
InVivoMAb Anti-DENV-2 Envelope protein E/EDIII (Iv0125) |
|
|
InVivoMAb Anti-DENV-2 Envelope protein E/EDIII (Iv0124) |
|
|
Recombinant DENV-1 Envelope protein E, N-His |
|
|
Recombinant DENV-4 Envelope protein E, N-His |
|
|
Recombinant DENV-2 Envelope protein E, N-His |
|
|
Recombinant DENV-3 Envelope protein E, N-His |
|
|
Recombinant DENV-2 Envelope protein E, N-His |
|
|
Anti-Dengue and Zika virus EDE1 Antibody (EDE1C10) |
|
|
Anti-DENV-2 E-DIII antibody (1A1D) |
References
1. Khetarpal N, Khanna I. Dengue Fever: Causes, Complications, and Vaccine Strategies. Journal of Immunology Research. 2016;2016:1-14. doi:https://doi.org/10.1155/2016/6803098
2. Parveen S, Riaz Z, Saeed S, et al. Dengue Hemorrhagic Fever: A Growing Global Menace. Journal of Water and Health. 2023;21(11). doi:https://doi.org/10.2166/wh.2023.114
3. Yan K, Mao L, Lan J, Xiao Z. Advancements in dengue vaccines: A historical overview and pro-spects for following next-generation candidates. Journal of Microbiology. 2025;63(2):e2410018. doi:https://doi.org/10.71150/jm.2410018
4. Khan MB, Yang ZS, Lin CC, et al. Dengue Overview: An Updated Systemic Review. Journal of Infection and Public Health. 2023;16(10). doi:https://doi.org/10.1016/j.jiph.2023.08.001
