Catalog No.
DHC27705
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
T3E, T-cell surface antigen T3/Leu-4 epsilon chain, CD3e, CD3E, T-cell surface glycoprotein CD3 epsilon chain
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
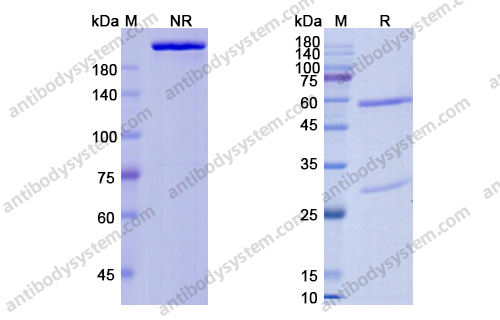
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P07766
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
NI-0401, CAS: 946415-64-1
Clone ID
Foralumab
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside, PMID: 27161438
Targeting T cells in inflammatory bowel disease, PMID: 32585338
Oral treatment with foralumab, a fully human anti-CD3 monoclonal antibody, prevents skin xenograft rejection in humanized mice, PMID: 28739191
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases, PMID: 23254986
Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH, PMID: 29920654
Preclinical efficacy of humanized, non-FcγR-binding anti-CD3 antibodies in T-cell acute lymphoblastic leukemia, PMID: 32483610
Dampening of Microglial Activation With Nasal Foralumab Administration in Moderate Alzheimer's Disease Dementia., PMID:40359013
Nasal administration of anti-CD3 mAb (Foralumab) downregulates NKG7 and increases TGFB1 and GIMAP7 expression in T cells in subjects with COVID-19., PMID:36881624
Flow Characteristics of the Conjugate of Anti-CD3 Monoclonal Antibodies and Magnetic Nanoparticle in PBS and Blood Vessels., PMID:36527662
Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects., PMID:36505477
Injection Effect of Anti-CD3 Monoclonal Antibody on Primo Vessel in Lymph Vessel of Rabbit with Lipopolysaccharide-Induced Inflammation., PMID:35770572
Corrigendum: Nasal Administration of Anti-CD3 Monoclonal Antibody (Foralumab) Reduces Lung Inflammation and Blood Inflammatory Biomarkers in Mild to Moderate COVID-19 Patients: A Pilot Study., PMID:35095916
Nasal Administration of Anti-CD3 Monoclonal Antibody (Foralumab) Reduces Lung Inflammation and Blood Inflammatory Biomarkers in Mild to Moderate COVID-19 Patients: A Pilot Study., PMID:34475873
Targeting T cells in inflammatory bowel disease., PMID:32585338
Preclinical efficacy of humanized, non-FcγR-binding anti-CD3 antibodies in T-cell acute lymphoblastic leukemia., PMID:32483610
Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH., PMID:29920654
Oral treatment with foralumab, a fully human anti-CD3 monoclonal antibody, prevents skin xenograft rejection in humanized mice., PMID:28739191
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside., PMID:27161438
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases., PMID:23254986