Catalog No.
DVV02822
Expression system
Mammalian Cells
Species reactivity
Human respiratory syncytial virus A (strain A2)
Host species
Human
Isotype
IgG1
Clonality
Monoclonal
Target
F, Fusion glycoprotein F0, Fusion glycoprotein F2, p27, Intervening segment, Pep27, Peptide 27, Fusion glycoprotein F1
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
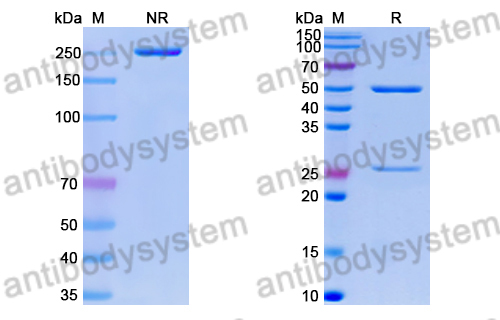
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P03420
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ADI-14442, ADI14442, ADI 14442
Clone ID
ADI-14442
Hydrophobic residue substitutions enhance the stability and in vivo immunogenicity of respiratory syncytial virus fusion protein., PMID:40434102
Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models., PMID:40432136
Strength and durability of RSV pre-fusion F IgG following infection and exposure in a household cohort, 2014-2022., PMID:40176487
Recovery of Antibody Immunity After a Resurgence of Respiratory Syncytial Virus Infections., PMID:40099894
A truncated pre-F protein mRNA vaccine elicits an enhanced immune response and protection against respiratory syncytial virus., PMID:39910047
Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus., PMID:39852872
Protective or limited? Maternal antibodies and RSV-associated lower respiratory tract infection in hospitalized infants aged 28-90 days., PMID:39845974
DS2 designer pre-fusion F vaccine induces strong and protective antibody response against RSV infection., PMID:39741146
Safety and immunogenicity of the Ad26/protein preF RSV vaccine in adults aged 18 to 59 years with and without at-risk comorbidities for severe respiratory syncytial virus disease: A phase 3, randomized, controlled, immunobridging trial., PMID:39536455
Serum and mucosal antibody-mediated protection and identification of asymptomatic respiratory syncytial virus infection in community-dwelling older adults in Europe., PMID:39493753
Novel intercellular spread mode of respiratory syncytial virus contributes to neutralization escape., PMID:39489301
Induction of neutralizing antibody responses by AAV5-based vaccine for respiratory syncytial virus in mice., PMID:39469716
RSV Neutralizing Antibodies Following Nirsevimab and Palivizumab Dosing., PMID:39350745
Mutating a flexible region of the RSV F protein can stabilize the prefusion conformation., PMID:39325881
Confronting the challenge: a regional perspective by the Latin American pediatric infectious diseases society (SLIPE) expert group on respiratory syncytial virus-tackling the burden of disease and implementing preventive solutions., PMID:39144471
Antibody levels against respiratory syncytial virus fusion protein conformations and lack of association with life-threatening infection in previously healthy infants., PMID:39003106
Development and Validation of an Enzyme-Linked Immunosorbent Assay-Based Protocol for Evaluation of Respiratory Syncytial Virus Vaccines., PMID:38932244
Antibody responses to respiratory syncytial virus: a population-based cross-sectional serological study in Southern China, 2021., PMID:38852851
Exacerbated lung inflammation in offspring with high maternal antibody levels following secondary RSV exposure., PMID:38745662
Intranasal respiratory syncytial virus vaccine attenuated by codon-pair deoptimization of seven open reading frames is genetically stable and elicits mucosal and systemic immunity and protection against challenge virus replication in hamsters., PMID:38739647
Mutations in the F protein of the live-attenuated respiratory syncytial virus vaccine candidate ΔNS2/Δ1313/I1314L increase the stability of infectivity and content of prefusion F protein., PMID:38593167
[Prevention of respiratory syncytial virus infection in infants. What has been done and where are we today?]., PMID:38329302
Assessing the protection elicited by virus-like particles expressing the RSV pre-fusion F and tandem repeated G proteins against RSV rA2 line19F infection in mice., PMID:38178222
Structure-based design of a single-chain triple-disulfide-stabilized fusion-glycoprotein trimer that elicits high-titer neutralizing responses against human metapneumovirus., PMID:37738240
Virus-Like Particles Assembled Using Respiratory Syncytial Virus Matrix Protein Elicit Protective Immunity in Mice., PMID:37719656
Development of Respiratory Syncytial Virus Vaccine Candidates for the Elderly., PMID:37376605
Gamma Irradiation-Inactivated Respiratory Syncytial Virus Vaccine Provides Protection but Exacerbates Pulmonary Inflammation by Switching from Prefusion to Postfusion F Protein., PMID:37272801
The Efficiency of p27 Cleavage during In Vitro Respiratory Syncytial Virus (RSV) Infection Is Cell Line and RSV Subtype Dependent., PMID:37133390
Development of a Kinetic ELISA and Reactive B Cell Frequency Assay to Detect Respiratory Syncytial Virus Pre-Fusion F Protein-Specific Immune Responses in Infants., PMID:37029694
Virus-like Particle Vaccine Expressing the Respiratory Syncytial Virus Pre-Fusion and G Proteins Confers Protection against RSV Challenge Infection., PMID:36986643
A human antibody potently neutralizes RSV by targeting the conserved hydrophobic region of prefusion F., PMID:36853487
The Respiratory Syncytial Virus (RSV) G Protein Enhances the Immune Responses to the RSV F Protein in an Enveloped Virus-Like Particle Vaccine Candidate., PMID:36602367
Virus-like particles containing a prefusion-stabilized F protein induce a balanced immune response and confer protection against respiratory syncytial virus infection in mice., PMID:36578490
A prefusion-stabilized RSV F subunit vaccine elicits B cell responses with greater breadth and potency than a postfusion F vaccine., PMID:36542692
RSV pre-fusion F protein enhances the G protein antibody and anti-infectious responses., PMID:36535957
An Optimized FI-RSV Vaccine Effectively Protects Cotton Rats and BALB/c Mice without Causing Enhanced Respiratory Disease., PMID:36298640
Split-Dose Administration Enhances Immune Responses Elicited by a mRNA/Lipid Nanoparticle Vaccine Expressing Respiratory Syncytial Virus F Protein., PMID:36251490
Divergent age-related humoral correlates of protection against respiratory syncytial virus infection in older and young adults: a pilot, controlled, human infection challenge model., PMID:36098319
Immunogenicity and protective efficacy of RSV G central conserved domain vaccine with a prefusion nanoparticle., PMID:35773301
Elicitation of pneumovirus-specific B cell responses by a prefusion-stabilized respiratory syncytial virus F subunit vaccine., PMID:35731888
Evolution of protection after maternal immunization for respiratory syncytial virus in cotton rats., PMID:34941963
COVID-19, Influenza and RSV: Surveillance-informed prevention and treatment - Meeting report from an isirv-WHO virtual conference., PMID:34933044
Respiratory Syncytial Virus (RSV)-Specific Antibodies in Pregnant Women and Subsequent Risk of RSV Hospitalization in Young Infants., PMID:34129040
Level of maternal respiratory syncytial virus (RSV) F antibodies in hospitalized children and correlates of protection., PMID:34118428
In silico virtual screening of lead compounds for major antigenic sites in respiratory syncytial virus fusion protein., PMID:33969268
Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial., PMID:33864736
Vaccination with prefusion-stabilized respiratory syncytial virus fusion protein induces genetically and antigenically diverse antibody responses., PMID:33823129
Prevention of Respiratory Syncytial Virus Infection in Healthy Adults by a Single Immunization of Ad26.RSV.preF in a Human Challenge Study., PMID:33400792
The respiratory syncytial virus fusion protein-specific B cell receptor repertoire reshaped by post-fusion subunit vaccination., PMID:33131932