Catalog No.
DHF14605
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IGHG1 Fc Fragment
Clonality
Monoclonal
Target
IgG Fc fragment receptor transporter alpha chain, IgG receptor FcRn large subunit p51, FcRn, FCGRT, FCRN, Neonatal Fc receptor
Concentration
4.1 mg/ml
Endotoxin level
Please contact with the lab for this information.
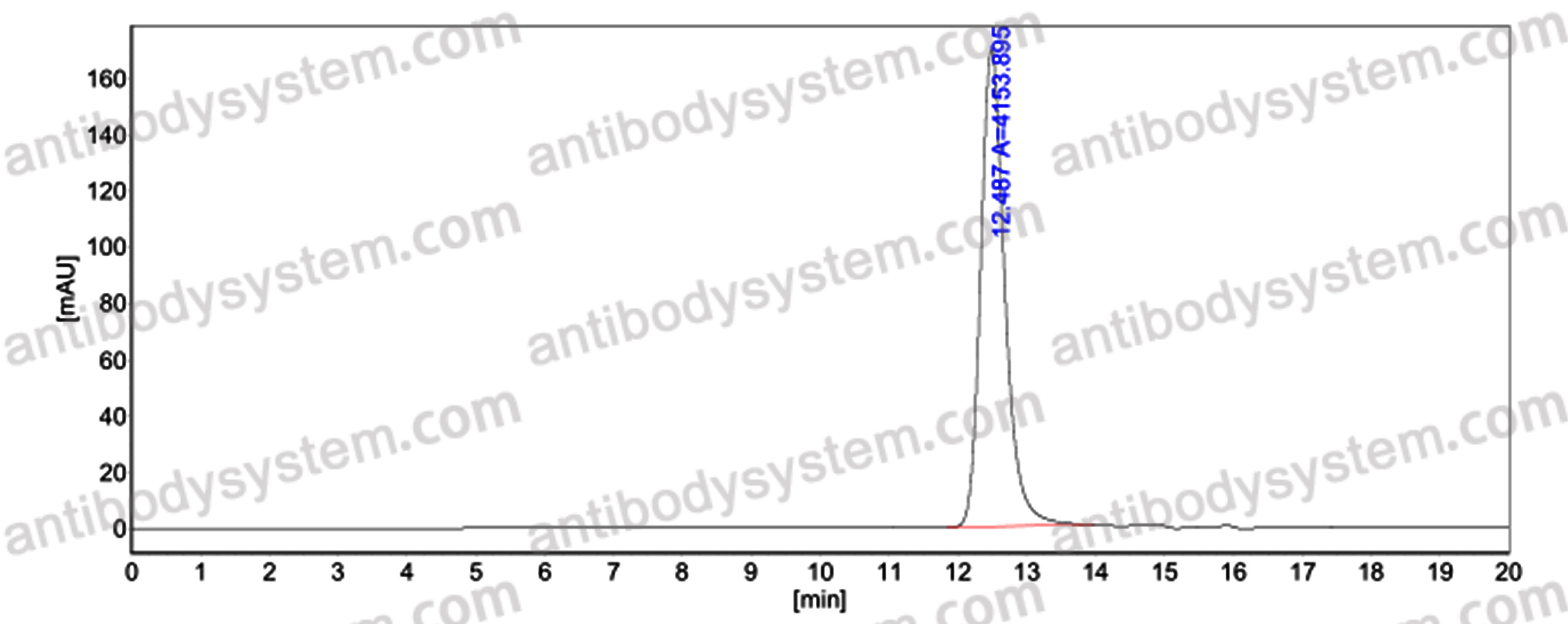
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P55899
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ARGX-113, ARGX 113, ARGX113, efgartigimod alfa-fcab
Clone ID
Efgartigimod
Pembrolizumab-induced myocarditis, myositis, and myasthenia gravis overlap syndrome (IM3OS) treated with Efgartigimod: case report., PMID:40510022
FcRn inhibitors in immune thrombocytopenia: A comprehensive review of therapeutic advances and clinical outcomes., PMID:40505332
Potential drug interaction between calcitonin gene-related peptide antagonists and neonatal Fc receptor inhibitors., PMID:40470831
Efgartigimod combined with steroids as a fast-acting therapy for anti-SRP immune-mediated necrotizing myopathy., PMID:40470493
Antibody-mediated Rejection - Treatment Standard., PMID:40440205
Correction: Fast-acting treatment of myasthenic crisis with efgartigimod from the perspective of the neuro intensive care unit., PMID:40413473
Real world study in Italian public hospital with Efgartigimod in patients affected by generalized myasthenia gravis: influence of clinical and serological factors., PMID:40401017
Efgartigimod Is an Effective Treatment for Triple-Seronegative Generalized Myasthenia Gravis: A Report of Three Patients., PMID:40391492
Short-term treatment of CIDP with efgartigimod: a case series in China., PMID:40375986
Resolution of anti-GAD-associated autoimmune encephalitis in patients treated with efgartigimod., PMID:40356636
An adolescent patient with anti-N-methyl-D-aspartate receptor encephalitis with motor aphasia as the first symptom and complicated by peripheral nerve damage: A case report and literature review., PMID:40355209
A cost analysis of reductions in work productivity for MG patients and their caregivers by symptom severity., PMID:40352835
Atypical Bulbar Myasthenia Gravis in an Elderly Male Unmasked by Levofloxacin: A Diagnostic Challenge., PMID:40352702
Efgartigimod for the treatment of Guillain-Barré syndrome with concurrent posterior reversible encephalopathy syndrome., PMID:40347748
Perioperative Safety and Efficacy of Efgartigimod for Thymoma-Associated Myasthenia Gravis: A Prospective, Multicenter, Phase II Clinical Trial., PMID:40320172
Efgartigimod as a fast-acting treatment in generalized very-late-onset myasthenia gravis., PMID:40313945
A real-world pharmacovigilance study of efgartigimod alfa in the FDA adverse event reporting system database., PMID:40308753
Correction: A real-life experience with eculizumab and efgartigimod in generalized myasthenia gravis patients., PMID:40299078
A pathophysiological and mechanistic review of chronic inflammatory demyelinating polyradiculoneuropathy therapy., PMID:40297573
Safety profile of efgartigimod from global clinical trials across multiple immunoglobulin G-mediated autoimmune diseases., PMID:40296516
Assessing the symptom control provided by ravulizumab or efgartigimod in myasthenia gravis: an evaluation of the patient experience of two different treatment approaches., PMID:40293925
Efficacy and safety of FcRn inhibitors in patients with Myasthenia gravis: An updated systematic review and meta‑analysis., PMID:40288289
Successful treatment of morvan syndrome with efgartigimod: report of two cases., PMID:40268813
Real-World Experience with FcRn Inhibitors Efgartigimod and Rozanolixizumab in Myasthenia Gravis: Administration in Multiple Cycles and Transition from Intravenous to Subcutaneous Formulation., PMID:40257679
Efficacy and Safety of Efgartigimod for Patients With Myasthenia Gravis in a Real-World Cohort of 77 Patients., PMID:40237260
Efgartigimod Followed by Telitacicept in Adult Generalized Myasthenia Gravis: A Retrospective Case Series., PMID:40224392
ADAPT NXT: Fixed Cycles or Every-Other-Week IV Efgartigimod in Generalized Myasthenia Gravis., PMID:40223516
Case Report: A myasthenia gravis patient complicated with renal failure was effectively treated with efgartigimod., PMID:40213565
Safety and effectiveness of efgartigimod for intravenous infusion in patients with generalized myasthenia gravis: an interim analysis of Japanese post-marketing surveillance., PMID:40200387
Telitacicept as an alternative to non-steroidal immunosuppressive therapies in the treatment of myasthenia gravis: a study on clinical efficacy and steroid-sparing effect., PMID:40196119
Efficacy of multi-cycle Efgartigimod in achieving minimal symptom expression in myasthenia gravis: A comparative multi-center study., PMID:40186904
Multicenter experience with Efgartigimod in the treatment of anti-NMDAR encephalitis compared with IVIG and SPA-IA during acute attacks., PMID:40180242
Efgartigimod for induction and maintenance therapy in muscle-specific kinase myasthenia gravis., PMID:40144041
Efgartigimod improves non-AChR generalized Myasthenia Gravis: a real world experience., PMID:40128464
Evaluating pharmacist preferences: Preparation of a novel on-body delivery system vs. high-resistance, manual syringes for large-volume subcutaneous drugs., PMID:40080876
The Impact of Efgartigimod on Utilization of Therapeutic Plasma Exchange Procedures for Myasthenia Gravis in One Tertiary Medical Center., PMID:40051346
[Combination therapy with efgartigimod, fostamatinib, eltrombopag and prednisolone for a patient with refractory immune thrombocytopenia]., PMID:40044172
Sustained Minimal Manifestations With Extended Interval Dosing of Efgartigimod in Seronegative Myasthenia Gravis: Case Report., PMID:40040605
Impact of FcRn antagonism on vaccine-induced protective immune responses against viral challenge in COVID-19 and influenza mouse vaccination models., PMID:40028815
A systematic review of efgartigimod as an effective treatment for myasthenic crisis., PMID:40014125
Sequential administration of efgartigimod shortened the course of Guillain-Barré syndrome: a case series., PMID:40012687
Fast-acting treatment of myasthenic crisis with efgartigimod from the perspective of the neonatal intensive care unit., PMID:40012057
Response of refractory residual ocular symptoms to efgartigimod in generalized myasthenia gravis: A real-world case series., PMID:39999710
The efficacy and safety between efgartigimod and intravenous immunoglobulin in elderly generalized myasthenia gravis patients., PMID:39988290
Patterns and predictors of therapeutic response to efgartigimod in acetylcholine receptor-antibody generalized myasthenia gravis subtypes., PMID:39974170
Effects of efgartigimod treatment on humoral and cellular immune responses: analysis of T-cell-dependent antibody response in cynomolgus monkeys., PMID:39945037
Efficacy and safety of efgartigimod in patients with neurological autoimmune diseases., PMID:39938695
Case report: Successful perioperative intervention with efgartigimod in a patient in myasthenic crisis., PMID:39935471
Bullous pemphigoid: A practical approach to diagnosis and management in the modern era., PMID:39914667