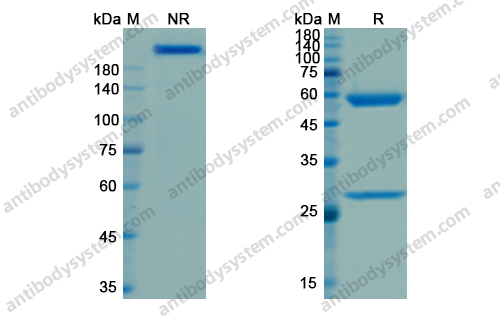
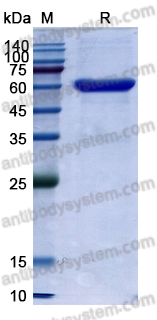
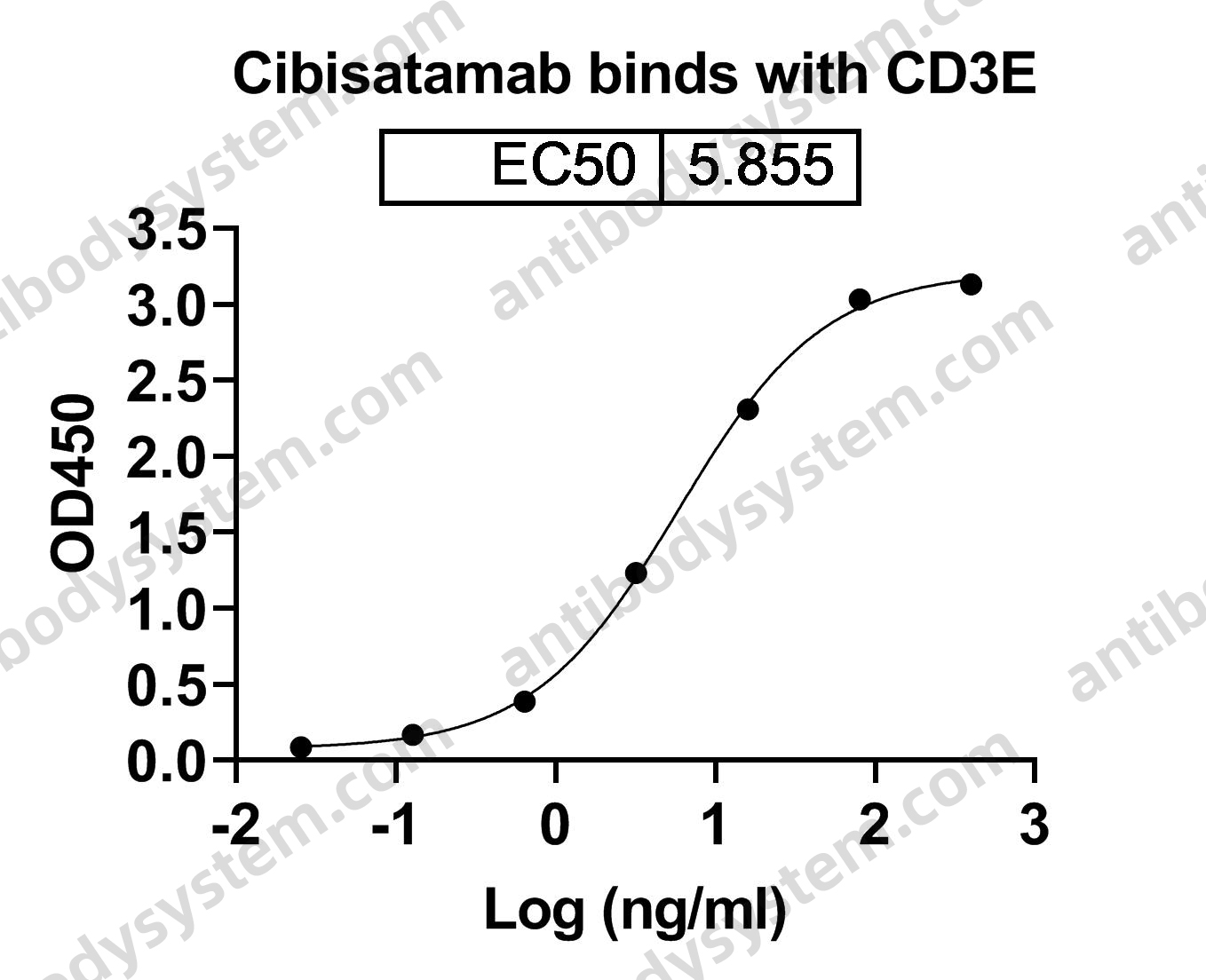
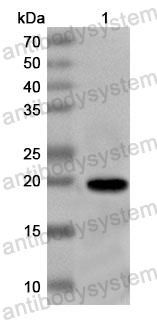
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside, PMID: 27161438
99m Tc-Labeled succinimidyl-6-hydrazinonicotinate hydrochloride (SHNH)-conjugated visilizumab, PMID: 20641817
Targeting T cells in inflammatory bowel disease, PMID: 32585338
Visilizumab induces apoptosis of mucosal T lymphocytes in ulcerative colitis through activation of caspase 3 and 8 dependent pathways, PMID: 18424236
Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis, PMID: 20947884
Visilizumab with tacrolimus and methotrexate for GvHD prevention after allogeneic hematopoietic cell transplantation from mismatched unrelated donors, PMID: 27991896
Inflammatory bowel disease: clinical aspects and established and evolving therapies, PMID: 17499606
Radiolabeled humanized anti-CD3 monoclonal antibody visilizumab for imaging human T-lymphocytes, PMID: 19759100
A humanized non-FcR-binding anti-CD3 antibody, visilizumab, for treatment of steroid-refractory acute graft-versus-host disease, PMID: 11929757
[Pyoderma gangrenosum treated successfully with visilizumab in patients with ulcerative colitis], PMID: 20071320
IBD: Visilizumab not useful for intravenous steroid-refractory ulcerative colitis, PMID: 21265059
A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis, PMID: 17920064
Prospective randomized open-label multicenter phase I/II dose escalation trial of visilizumab (HuM291) in severe steroid-refractory ulcerative colitis, PMID: 19714757
A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease, PMID: 15931635
Transient cytokine-induced liver injury following administration of the humanized anti-CD3 antibody visilizumab (HuM291) in Crohn's disease, PMID: 19240707
Assessing the risk of epstein-barr virus-related lymphoproliferative disorders before administration of visilizumab, PMID: 18325408
Pyoderma gangraenosum, PMID: 21037478
Emerging treatments for ulcerative colitis: a systematic review, PMID: 28503977
Visilizumab in severe ulcerative colitis: good vision but blurry results, PMID: 18383522
Immunotherapy in inflammatory bowel disease, PMID: 22703854
Management of acute severe colitis, PMID: 16847166
Anti-CD3 mAbs for treatment of type 1 diabetes, PMID: 19319985
Advances in medical therapy of inflammatory bowel disease, PMID: 16213789
Advances in biologic therapy for ulcerative colitis and Crohn's disease, PMID: 17105690
Therapy of ulcerative colitis: state of the art, PMID: 18622161
Monoclonal antibodies for the prevention and treatment of graft-versus-host disease, PMID: 12939720
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases, PMID: 23254986
Therapeutic options in acute severe ulcerative colitis, PMID: 19072385
Steroid-refractory graft-vs.-host disease: past, present and future, PMID: 12603689
The use of monoclonal antibodies in immune-mediated hematologic disorders, PMID: 22703857
IBD in 2010: optimizing treatment and minimizing adverse events, PMID: 21293506
Update in medical therapy of ulcerative colitis: newer concepts and therapies, PMID: 16000921
Biological agents for ulcerative colitis: hypes and hopes, PMID: 17464967
Novel therapeutics for the treatment of graft-versus-host disease, PMID: 12225248
Systematic review of the effectiveness of biological therapy for active moderate to severe ulcerative colitis, PMID: 24955447
Gateways to clinical trials, PMID: 21069103
General principles and pharmacology of biologics in inflammatory bowel disease, PMID: 17129812
Gateways to clinical trials, PMID: 16082427
Gateways to clinical trials, PMID: 12949633
Current state of type 1 diabetes immunotherapy: incremental advances, huge leaps, or more of the same?, PMID: 21785616
Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8 + T cells in early clinical stages of chronic lymphocytic leukemia, PMID: 32710227
Gateways to clinical trials, PMID: 12224444
Medical therapy for ulcerative colitis: the state of the art and beyond, PMID: 15527679
Review article: saving the colon in severe colitis - the case for medical therapy, PMID: 16961749
Can super-antibody drugs be tamed?, PMID: 16612349
In vitro analyses of the immunosuppressive properties of neural stem/progenitor cells using anti-CD3/CD28-activated T cells, PMID: 20941615
Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice, PMID: 24403315
[The role of translational research in inflammatory bowel disease], PMID: 17966376
[Biotherapy of auto-immune diseases : past, present and future perspectives], PMID: 19303737
Tissue repair and ulcer/wound healing - Institut Pasteur Euroconference: molecular mechanisms, therapeutic targets and future directions, PMID: 15883915
Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8+ T cells in early clinical stages of chronic lymphocytic leukemia., PMID:32710227
Targeting T cells in inflammatory bowel disease., PMID:32585338
Emerging treatments for ulcerative colitis: a systematic review., PMID:28503977
Visilizumab with tacrolimus and methotrexate for GvHD prevention after allogeneic hematopoietic cell transplantation from mismatched unrelated donors., PMID:27991896
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside., PMID:27161438
Heterogeneous CD3 expression levels in differing T cell subsets correlate with the in vivo anti-CD3-mediated T cell modulation., PMID:25646305
Systematic review of the effectiveness of biological therapy for active moderate to severe ulcerative colitis., PMID:24955447
Fine tuning effector and regulatory T-cell dynamics: a novel tool for plaque regression?, PMID:24556952
Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice., PMID:24403315
Delayed anti-CD3 therapy results in depletion of alloreactive T cells and the dominance of Foxp3+ CD4+ graft infiltrating cells., PMID:23750800
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases., PMID:23254986
The use of monoclonal antibodies in immune-mediated hematologic disorders., PMID:22703857
Immunotherapy in inflammatory bowel disease., PMID:22703854
Current state of type 1 diabetes immunotherapy: incremental advances, huge leaps, or more of the same?, PMID:21785616
Oral anti-CD3 monoclonal antibody delays diabetes in non-obese diabetic (NOD) mice: effects on pregnancy and offspring--a preliminary report., PMID:21484981
IBD in 2010: optimizing treatment and minimizing adverse events., PMID:21293506
IBD: Visilizumab not useful for intravenous steroid-refractory ulcerative colitis., PMID:21265059
Gateways to clinical trials., PMID:21069103
Pyoderma gangraenosum., PMID:21037478
Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis., PMID:20947884
In vitro analyses of the immunosuppressive properties of neural stem/progenitor cells using anti-CD3/CD28-activated T cells., PMID:20941615
[Pyoderma gangrenosum treated successfully with visilizumab in patients with ulcerative colitis]., PMID:20071320
Radiolabeled humanized anti-CD3 monoclonal antibody visilizumab for imaging human T-lymphocytes., PMID:19759100
Prospective randomized open-label multicenter phase I/II dose escalation trial of visilizumab (HuM291) in severe steroid-refractory ulcerative colitis., PMID:19714757
Anti-CD3 mAbs for treatment of type 1 diabetes., PMID:19319985
[Biotherapy of auto-immune diseases : past, present and future perspectives]., PMID:19303737
Transient cytokine-induced liver injury following administration of the humanized anti-CD3 antibody visilizumab (HuM291) in Crohn's disease., PMID:19240707
Therapeutic options in acute severe ulcerative colitis., PMID:19072385
DNA repair after DNA fragmentation in mouse small intestinal epithelial cells., PMID:19015882
Therapy of ulcerative colitis: state of the art., PMID:18622161
[The role of extracellular domain of human 4-1BBL in regulating activities of human peripheral blood lymphocytes in vitro]., PMID:18466693
Visilizumab induces apoptosis of mucosal T lymphocytes in ulcerative colitis through activation of caspase 3 and 8 dependent pathways., PMID:18424236
Visilizumab in severe ulcerative colitis: good vision but blurry results., PMID:18383522
Assessing the risk of epstein-barr virus-related lymphoproliferative disorders before administration of visilizumab., PMID:18325408
[The role of translational research in inflammatory bowel disease]., PMID:17966376
A phase I study of visilizumab, a humanized anti-CD3 monoclonal antibody, in severe steroid-refractory ulcerative colitis., PMID:17920064
Inflammatory bowel disease: clinical aspects and established and evolving therapies., PMID:17499606
Biological agents for ulcerative colitis: hypes and hopes., PMID:17464967
General principles and pharmacology of biologics in inflammatory bowel disease., PMID:17129812
Advances in biologic therapy for ulcerative colitis and Crohn's disease., PMID:17105690
Review article: saving the colon in severe colitis - the case for medical therapy., PMID:16961749
Novel biological therapies for inflammatory bowel disease., PMID:16901384
Management of acute severe colitis., PMID:16847166
Digestive Disease Week 2006. Therapies for Crohn's disease and ulcerative colitis. 21-15 May 2006, Los Angeles, CA, USA., PMID:16821150
Can super-antibody drugs be tamed?, PMID:16612349
Advances in medical therapy of inflammatory bowel disease., PMID:16213789
Gateways to clinical trials., PMID:16082427
Update in medical therapy of ulcerative colitis: newer concepts and therapies., PMID:16000921
A phase II multicenter study of visilizumab, humanized anti-CD3 antibody, to treat steroid-refractory acute graft-versus-host disease., PMID:15931635