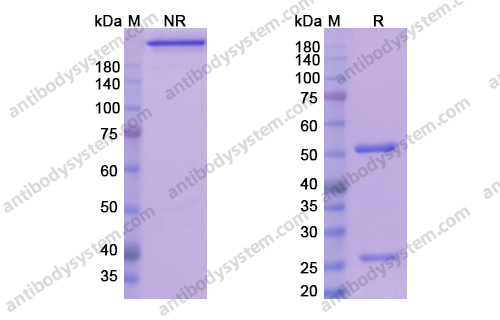
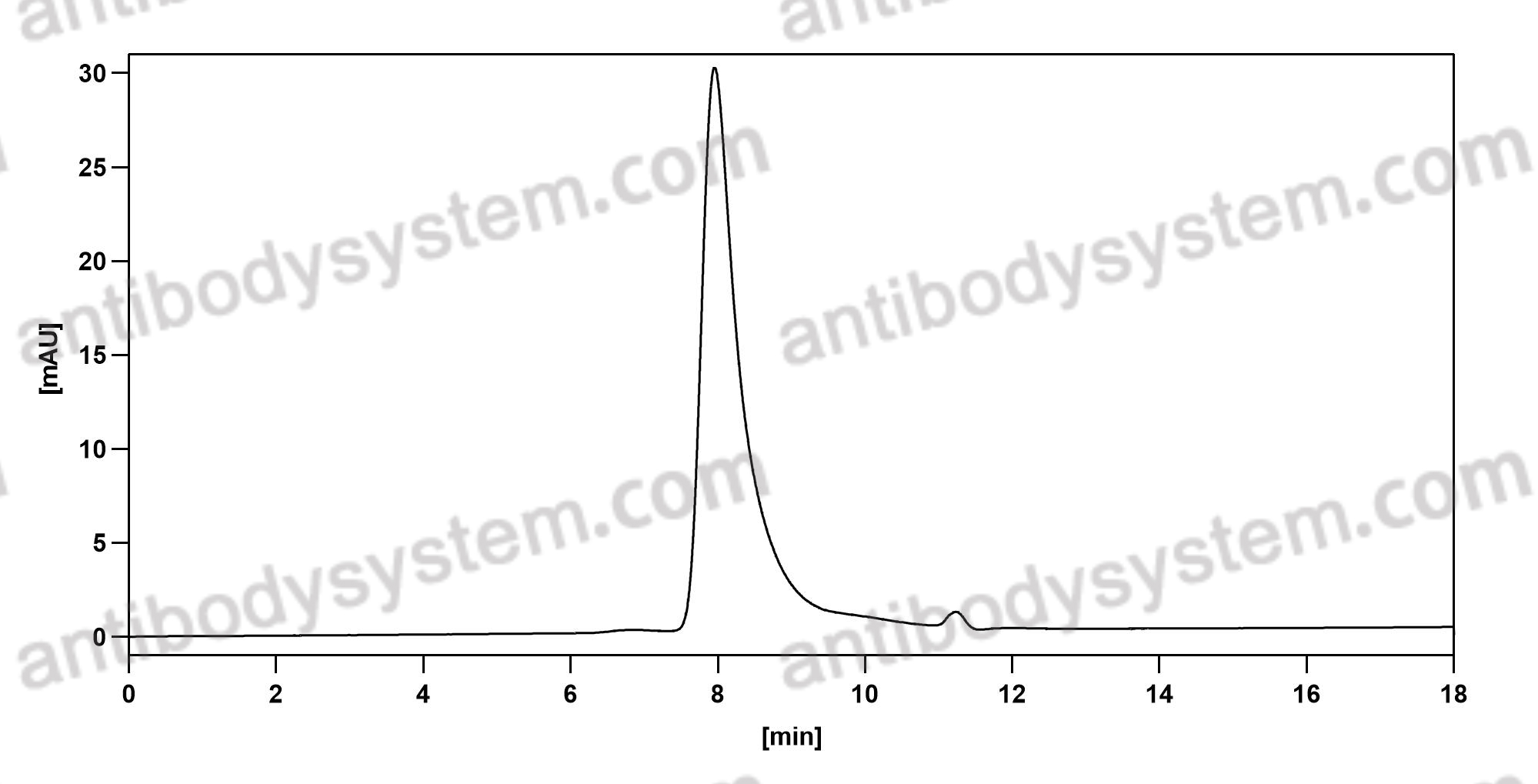
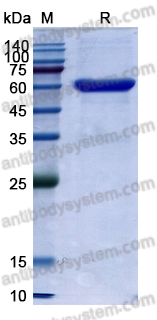
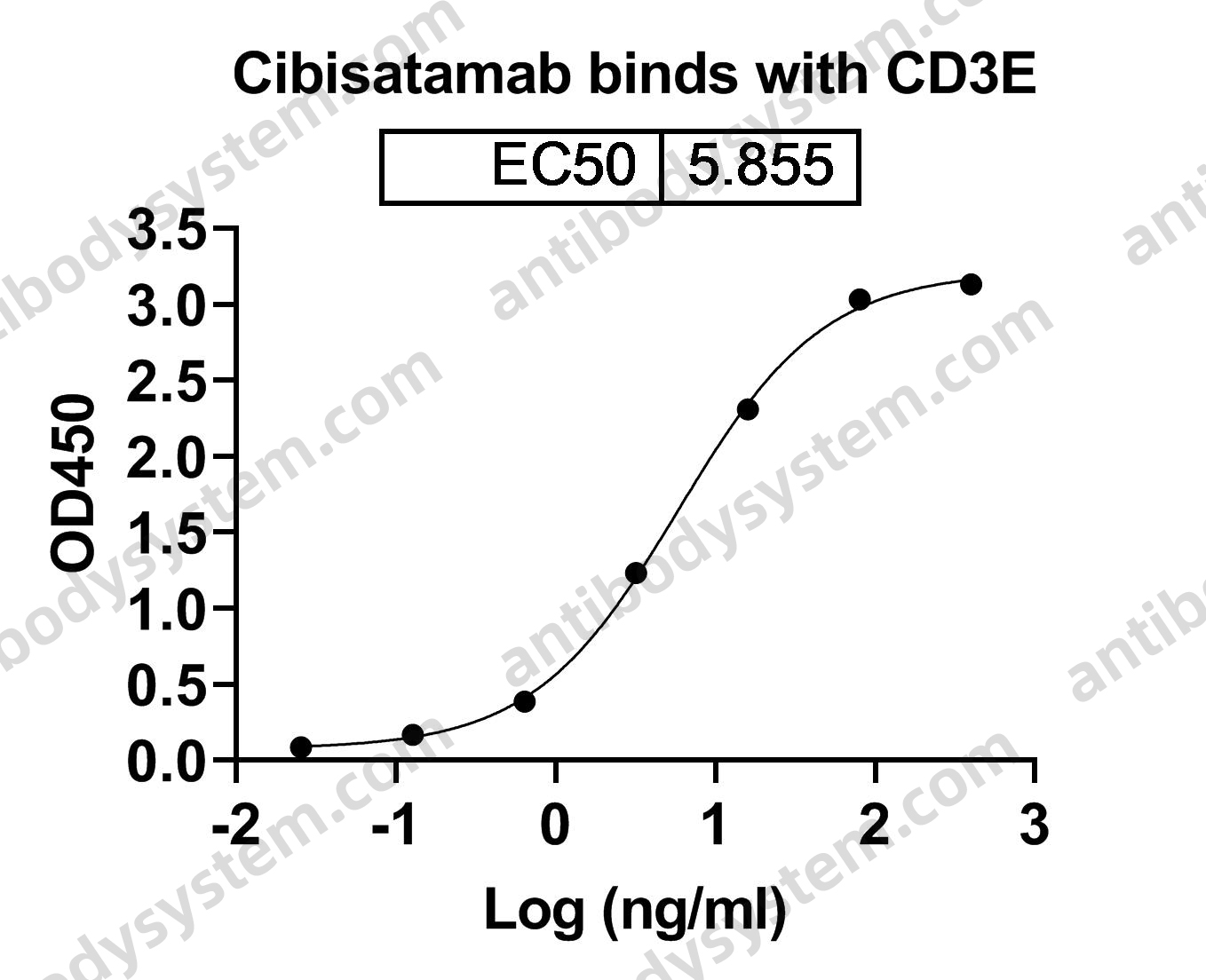
An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes, PMID: 31180194
Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial, PMID: 21719095
Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders, PMID: 23835333
Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis, PMID: 30569273
Antibodies to watch in 2020, PMID: 31847708
Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes, PMID: 28664195
Teplizumab for treatment of type 1 diabetes mellitus, PMID: 22968521
Teplizumab in Relatives at Risk for Type 1 Diabetes, PMID: 31693815
Teplizumab in Relatives at Risk for Type 1 Diabetes, PMID: 31693816
Teplizumab in Relatives at Risk for Type 1 Diabetes, PMID: 31693817
Teplizumab therapy for type 1 diabetes, PMID: 20095914
Teplizumab in Relatives at Risk for Type 1 Diabetes. Reply, PMID: 31693818
New Frontiers in the Treatment of Type 1 Diabetes, PMID: 31839487
Teplizumab delays onset of type 1 diabetes mellitus, PMID: 31243389
Safety and Efficacy of Teplizumab for Treatment of Type One Diabetes Mellitus: A Systematic Review and Meta-analysis, PMID: 33302842
Cost Effectiveness of Teplizumab for Prevention of Type 1 Diabetes Among Different Target Patient Groups, PMID: 32960433
Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside, PMID: 27161438
An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes, PMID: 32023396
Changing the landscape for type 1 diabetes: the first step to prevention, PMID: 31533907
Treatment of new onset type 1 diabetes with teplizumab: successes and pitfalls in development, PMID: 24517093
Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals, PMID: 33658358
Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial, PMID: 23086558
Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial, PMID: 23801579
Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients, PMID: 22277969
Teplizumab Improves Beta Cell Function, Delays Type 1 Diabetes, PMID: 33847731
Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes, PMID: 26518356
Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years, PMID: 19443276
Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes, PMID: 32833543
Prospects for therapeutic tolerance in humans, PMID: 24378931
Insulin is necessary but not sufficient: changing the therapeutic paradigm in type 1 diabetes, PMID: 32789003
Immunotherapies currently in development for the treatment of type 1 diabetes, PMID: 26364507
Immunotherapies in diabetes mellitus type 1, PMID: 22703858
Microbiota control immune regulation in humanized mice, PMID: 29093268
Anti-CD3 drug keeps diabetes at bay, PMID: 31578500
Combination therapies in the context of anti-CD3 antibodies for the treatment of autoimmune diseases, PMID: 23254986
Traveling down the Long Road to Type 1 Diabetes Mellitus Prevention, PMID: 31180193
Enforcing the checkpoints: harnessing T-cell exhaustion for therapy of T1D, PMID: 31157632
Anti-CD3 clinical trials in type 1 diabetes mellitus, PMID: 23726024
Anti-CD3 Antibody for the Prevention of Type 1 Diabetes: A Story of Perseverance, PMID: 31523950
Prevention versus intervention of type 1 diabetes, PMID: 23803322
Preclinical efficacy of humanized, non-FcγR-binding anti-CD3 antibodies in T-cell acute lymphoblastic leukemia, PMID: 32483610
Primary and secondary prevention of Type 1 diabetes, PMID: 23231526
Remodeling T cell compartments during anti-CD3 immunotherapy of type 1 diabetes, PMID: 28844471
Immune therapy and β-cell death in type 1 diabetes, PMID: 23423576
Immunomodulators: Cell savers, PMID: 22616095
Solving the Puzzle of Immune Tolerance for β-Cell Replacement Therapy for Type 1 Diabetes, PMID: 33007232
Lessons from type 1 diabetes for understanding natural history and prevention of autoimmune disease, PMID: 25437293
Anti-CD3 mAbs for treatment of type 1 diabetes, PMID: 19319985
Type 1 diabetes pathogenesis - Prevention???, PMID: 25941654
A future for CD3 antibodies in immunotherapy of type 1 diabetes, PMID: 30612137
Type 1 diabetes mellitus prevention: present and future., PMID:40527975
Latent EBV enhances the efficacy of anti-CD3 mAb in Type 1 diabetes., PMID:40447640
The Use of Continuous Glucose Monitoring to Diagnose Stage 2 Type 1 Diabetes., PMID:40444471
The Role of the Diabetes Care and Education Specialist in Screening and Monitoring for Type 1 Diabetes., PMID:40411371
Teplizumab: a promising intervention for delaying type 1 diabetes progression., PMID:40357199
An Exploratory Cost-Effectiveness Analysis of Immune Therapy in Delaying the Initiation of Automated Insulin Delivery Systems in Type 1 Diabetes., PMID:40354170
Paediatric screening in Italy as a gateway to secondary prevention in type 1 diabetes., PMID:40339706
Teplizumab: Not all that glitters is gold., PMID:40329663
Quality of life and physical activity in type 1 diabetes., PMID:40312313
Immunomodulatory agents and cell therapy for patients with type 1 diabetes., PMID:40215356
Immunotherapy as a treatment for type 1 diabetes mellitus in children and young adults: A comprehensive systematic review and meta-analysis., PMID:40215252
M120 Risk Score Improves Identification of Children at High Risk of Developing Clinical Type 1 Diabetes and Reports Short-Term Response to Preventive Immunotherapy., PMID:40208750
Pharmacotherapeutic strategies to promote regulatory T cell function in autoimmunity., PMID:40187268
Type 1 diabetes risk factors, risk prediction and presymptomatic detection: Evidence and guidance for screening., PMID:40134221
Anti-diabetic Biologicals: Exploring the Role of Different Analytical Techniques., PMID:40088445
Pharmacotherapy of type 1 diabetes - part 2 Today., PMID:40082213
Early expansion of TIGIT+PD1+ effector memory CD4 T cells via agonistic effect of alefacept in new-onset type 1 diabetes., PMID:40073269
Pharmacotherapy of type 1 diabetes - part 3: tomorrow., PMID:40056035
Immunotherapies for prevention and treatment of type 1 diabetes., PMID:40033931
[Early diagnosis of type 1 diabetes : a step towards precision medicine]., PMID:39976138
Real-world experiences of adult individuals or caregivers of children who received teplizumab treatment in stage 2 type 1 diabetes., PMID:39949173
Immunotherapy in Type 1 Diabetes: Emerging Therapies and Future Directions., PMID:39948801
Corrigendum: Shifting the paradigm of type 1 diabetes: a narrative review of disease-modifying therapies., PMID:39936148
Clinical Immunologic Interventions for the Treatment of Type 1 Diabetes: Challenges, Choice, and Timing of Immunomodulators., PMID:39929732
Emerging Immunotherapies for Disease Modification of Type 1 Diabetes., PMID:39873914
Pre-Type 1 Diabetes in Adolescents and Teens: Screening, Nutritional Interventions, Beta-Cell Preservation, and Psychosocial Impacts., PMID:39860389
An Atypical Presentation of Cytokine Release Syndrome With Signs of Arthritis During Treatment With Teplizumab in a Pediatric Patient., PMID:39841557
Current perspectives and the future of disease-modifying therapies in type 1 diabetes., PMID:39817218
Identifying Promising Immunomodulators for Type 1 Diabetes (T1D) and Islet Transplantation., PMID:39735417
ISPAD Clinical Practice Consensus Guidelines 2024: Screening, Staging, and Strategies to Preserve Beta-Cell Function in Children and Adolescents with Type 1 Diabetes., PMID:39662065
Time to reframe the disease staging system for type 1 diabetes., PMID:39608963
Shifting the paradigm of type 1 diabetes: a narrative review of disease modifying therapies., PMID:39568817
Trajectory of beta cell function and insulin clearance in stage 2 type 1 diabetes: natural history and response to teplizumab., PMID:39560746
Immunotherapy in type 1 diabetes: Novel pathway to the future ahead., PMID:39493558
Type 1 diabetes: immune pathology and novel therapeutic approaches., PMID:39469552
Latent Autoimmune Diabetes in Adults Following Bariatric Surgery-Induced Hypoglycemia in a 36-Year-Old Woman., PMID:39463547
Emerging Concepts and Success Stories in Type 1 Diabetes Research: A Road Map for a Bright Future., PMID:39446565
Immunotherapy-Based Strategies for Treatment of Type 1 Diabetes., PMID:39401495
The Benefits of Using Continuous Glucose Monitoring to Diagnose Type 1 Diabetes., PMID:39394887
The Teplizumab Saga: The Challenge of Not Getting Lost in Clinical Translation., PMID:39284671
Safety of teplizumab in patients with high-risk for diabetes mellitus type 1: A systematic review., PMID:39192866
Evolving Concepts in Pathophysiology, Screening, and Prevention of Type 1 Diabetes: Report of Diabetes Mellitus Interagency Coordinating Committee Workshop., PMID:39167668
From pre-clinical efficacy to promising clinical trials that delay Type 1 diabetes., PMID:39142538
Teplizumab induces persistent changes in the antigen-specific repertoire in individuals at risk for type 1 diabetes., PMID:39137044
The Pathogenesis of Type 1 Diabetes., PMID:39134388
Teplizumab's immunomodulatory effects on pancreatic β-cell function in type 1 diabetes mellitus., PMID:39123252
Exploring Novel Treatment Modalities for Type 1 Diabetes Mellitus: Potential and Prospects., PMID:39120188
Evaluation of teplizumab's efficacy and safety in treatment of type 1 diabetes mellitus: A systematic review and meta-analysis., PMID:39099823
The Role of Teplizumab in Newly Diagnosed Type 1 Diabetes., PMID:38992798
Reply to the Letter to the Editor, Endocrine Practice for "Role of Teplizumab, a Humanized Anti-CD3 Monoclonal Antibody, in Managing Newly Diagnosed Type 1 Diabetes: An Updated Systematic Review and Meta-Analysis"., PMID:38992797