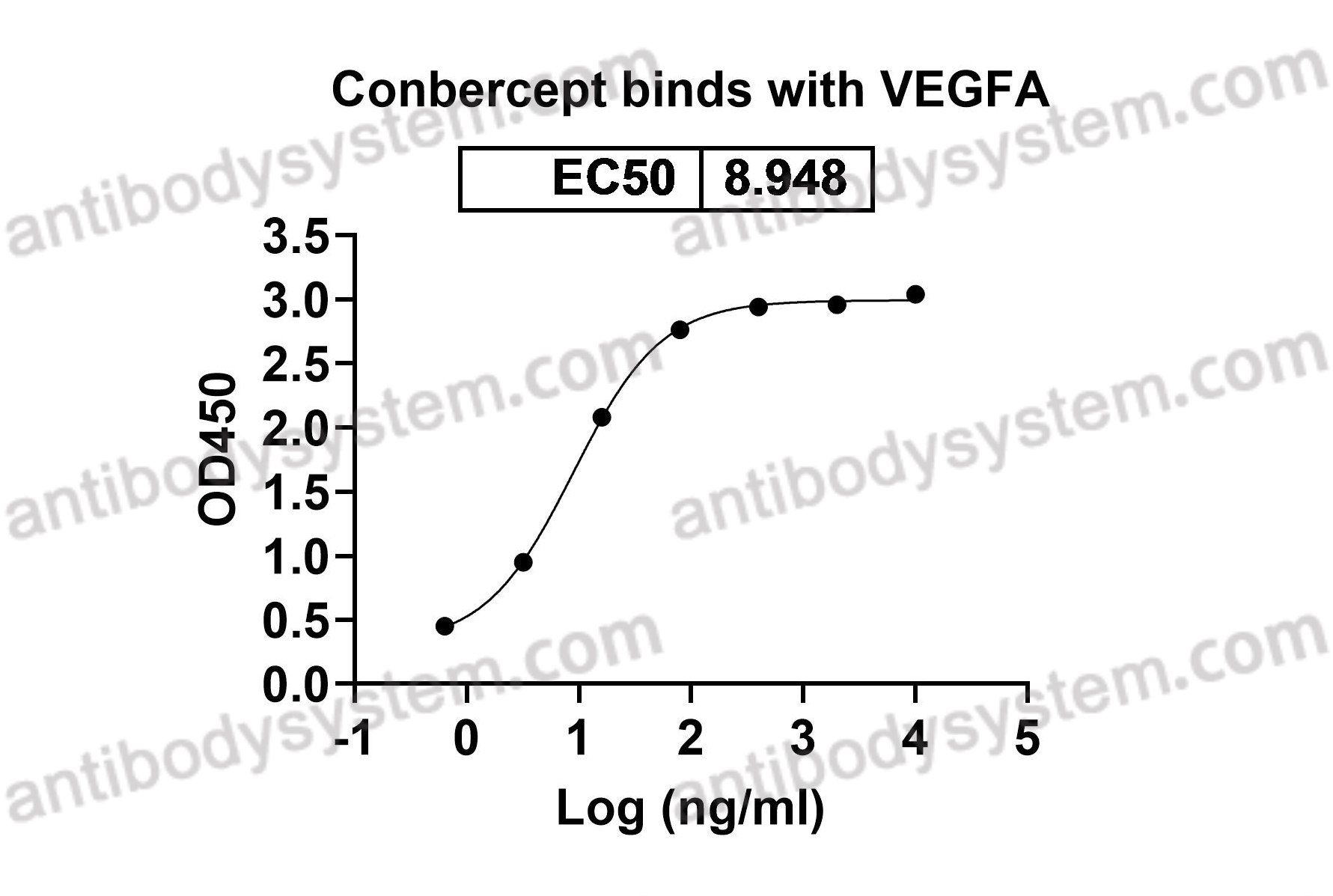
Catalog No.
DHD12608
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
Fusion - [FLT1 (fms-related tyrosine kinase 1, vascular endothelial growth factor receptor 1, VEGFR-1, VEGFR, FLT, FRT, vascular permeability factor receptor) - KDR (kinase insert domain receptor, vascular endothelial growth factor receptor 2, VEGFR2, VEGF-R2, FLK1, CD309)]2 - IGHG1 Fc (Fragment constant)
Target
Vascular endothelial growth factor A, VPF, VEGFA, VEGF, Vascular permeability factor, VEGF-A
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P15692
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
FP3 protein,KH902,KH903, CAS: 1227158-72-6
Clone ID
Conbercept
Ocular pharmacokinetics of intravitreal conbercept in a rabbit model following retinal scatter laser photocoagulation., PMID:40260388
Anti-Vascular Endothelial Growth Factor Crunch Syndrome in Proliferative Diabetic Retinopathy., PMID:40255438
Re: Cheng et al.: Conbercept versus laser for the treatment of infants with zone II retinopathy of prematurity (Ophthalmology. 2024;131:636-638)., PMID:40208175
Acute Corneal Epithelial Detachment During Lid Speculum Placement Prior to Intravitreal Injection in a Diabetic Patient: A Case Report., PMID:40201718
Intravitreal conbercept for chronic central serous chorioretinopathy with occult CNV: a retrospective clinical study based on multimodal ophthalmic imaging., PMID:40201323
Comparative efficacy of conbercept 3+PRN versus 3+TAE regimens for the treatment of polypoidal choroidal vasculopathy., PMID:40167859
Simultaneous inhibition of fibroblast growth factor-2 and vascular endothelial growth factor-a with RC28-E in diabetic macular edema: a phase 2 randomised trial., PMID:40122579
Renal dysfunction associated with clinical response to intravitreal conbercept therapy for diabetic macular edema., PMID:40103962
Serologic parameters in young patients with retinal vein occlusion treated with anti-vascular endothelial growth factor., PMID:40103956
Treat-And-Extend Versus Pro Re Nata Regimen of Intravitreal Conbercept Injection for Neovascular Age-Related Macular Degeneration: Results from COCOA, a Prospective, Open-Label, Multicenter, Randomized Phase IV Clinical Trial., PMID:40079155
Sozinibercept Combination Therapy for Neovascular Age-related Macular Degeneration: Phase 2b Study Subgroup Analysis by Lesion Type., PMID:39999360
Effect and factors associated with reactivation after intravitreal conbercept or aflibercept in retinopathy of prematurity., PMID:39871372
Inflammation and Occlusive Retinal Vasculitis Post Faricimab., PMID:39847370
A Novel Bispecific Anti-IL17/VEGF Fusion Trap Exhibits Potent and Long-Lasting Inhibitory Effects on the Development of Age-Related Macular Degeneration., PMID:39735855
Phase 1b Dose Escalation Study of Sozinibercept Inhibition of Vascular Endothelial Growth Factors C and D With Aflibercept for Diabetic Macular Edema., PMID:39699889
Retrospective analysis of the etiology and drugs for vitreous hemorrhage caused by non-diabetic retinopathy and non-traumatic factors., PMID:39592112
Short-term fluctuation of intraocular pressure and influencing factors following intravitreal injection in patients with retinal vascular diseases., PMID:39559303
The analysis of foveal microvascular anomalies in retinopathy of prematurity after anti-vascular endothelial growth factor therapy using optical coherence tomography angiography., PMID:39558298
Recurrence of Retinopathy of Prematurity Following Anti-vascular Endothelial Growth Factor (Anti-VEGF) Therapy: A Systematic Review and Meta-Analysis., PMID:39524166
Efficacy and safety of intravitreal conbercept and triamcinolone acetonide for wet age-related macular degeneration in China: a meta-analysis., PMID:39527325
Proton beam irradiation with anti-VEGF therapy for polypoidal choroidal vasculopathy: results of a 24-month, phase II randomized study., PMID:39520549
INTRAVITREAL INJECTION CONBERCEPT IMPROVES THE BEST-CORRECTED VISUAL ACUITY IN PATIENTS WITH WET AGE-RELATED MACULAR EDEMA., PMID:39441289
Vascular endothelial growth factor/connective tissue growth factor and proteomic analysis of aqueous humor after intravitreal conbercept for proliferative diabetes retinopathy., PMID:39430009
Two-year outcomes of intravitreal conbercept therapy for polypoidal choroidal vasculopathy., PMID:39379869
Evaluating the Efficacy of Conbercept and Dexamethasone Implants Sequentially in the Treatment of Refractory Macular Edema Secondary to Central Retinal Vein Occlusion (CRVO): A One-Year Follow-Up Study., PMID:39372223
Preoperative Intravitreal Conbercept Injection Reduced Both Angiogenic and Inflammatory Cytokines in Patients With Proliferative Diabetic Retinopathy., PMID:39308630
[Conbercept reverses TGF-β2-induced epithelial-mesenchymal transition in human lens epithelial cells by regulating the TGF-β/Smad signaling pathway]., PMID:39276041
Combined treatment of submacular hemorrhage with low-dose subretinal recombinant tissue plasminogen activator and intravitreal conbercept., PMID:39237907
VEGFA may be a potential marker of myopic choroidal thickness and vascular density changes., PMID:39227639
Comparison of ranibizumab and conbercept treatment in type 1 prethreshold retinopathy of prematurity in zone II., PMID:39215256
Multimodal imaging diagnosis and analysis of prognostic factors in patients with adult-onset Coats disease., PMID:39156792
Clinical and genetic features in autosomal recessive bestrophinopathy in Chinese cohort., PMID:39048936
Intravitreal Injection of Conbercept Combined with Dexamethasone for Macular Edema Following Central Retinal Vein Occlusion., PMID:38948340
Prognostic factors for intravitreal conbercept in the treatment of choroidal neovascularization secondary to pathological myopia., PMID:38907787
Intravitreal conbercept injection with panretinal photocoagulation for high-risk proliferative diabetic retinopathy with vitreous hemorrhage., PMID:38895681
Comparative efficacy of anti-vascular endothelial growth factor on diabetic macular edema diagnosed with different patterns of optical coherence tomography: A network meta-analysis., PMID:38848379
Efficacy and safety of intravitreal injection of conbercept for moderate to severe nonproliferative diabetic retinopathy., PMID:38846145
Comparative study on the efficacy of Conbercept and Aflibercept in the treatment of neovascular age-related macular degeneration., PMID:38796619
Association of the Complement Factor H Y402H Polymorphism and Response to Anti-Vascular Endothelial Growth Factor Treatment in Age-Related Macular Degeneration: An Updated Meta-Analysis., PMID:38754401
The efficacy of adjunctive mitomycin C and/or anti-VEGF agents on glaucoma tube shunt drainage device surgeries: a systematic review., PMID:38656422
Comparative quantitative analysis of optical coherence tomography angiography in varied morphologies of macular neovascularization following intravitreal conbercept and ranibizumab treatments for neovascular age‑related macular degeneration., PMID:38590577
Clinical effects of atorvastatin combined with conbercept in the treatment of patients with macular edema secondary to retinal vein occlusion and carotid plaque: study protocol for a prospective randomized controlled trial., PMID:38589960
Analysis aqueous humor lipid profile of neovascular glaucoma secondary to diabetic retinopathy and lipidomic alteration response to anti-VEGF treatment., PMID:38554799
The Anatomic and Functional Outcomes of Ozurdex-Aided Vitrectomy in Proliferative Diabetic Retinopathy., PMID:38476345
Clinical evaluation of ranibizumab and conbercept for the treatment of macular edema secondary to central retinal-vein occlusion., PMID:38448288
Comparative efficacy and safety of different anti-VEGF agents combined with different delivery methods for neovascular glaucoma: a systematic review and Bayesian network meta-analysis., PMID:38443085
MULTIPLE CHOROIDAL NEOVASCULARIZATIONS IN CHOROIDAL OSTEOMA TREATED WITH ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR INJECTIONS: A 6-YEAR FOLLOW-UP CASE REPORT., PMID:38442419
Comparison of different agents and doses of anti-vascular endothelial growth factors (aflibercept, bevacizumab, conbercept, ranibizumab) versus laser for retinopathy of prematurity: A network meta-analysis., PMID:38432359
Topical Ophthalmic Liposomes Dual-Modified with Penetratin and Hyaluronic Acid for the Noninvasive Treatment of Neovascular Age-Related Macular Degeneration., PMID:38414529
Efficacy and safety of intravitreal injections of conbercept for the treatment of idiopathic choroidal neovascularization., PMID:38373901