Catalog No.
DHC20803
Expression system
Mammalian Cells
Species reactivity
Human
Isotype
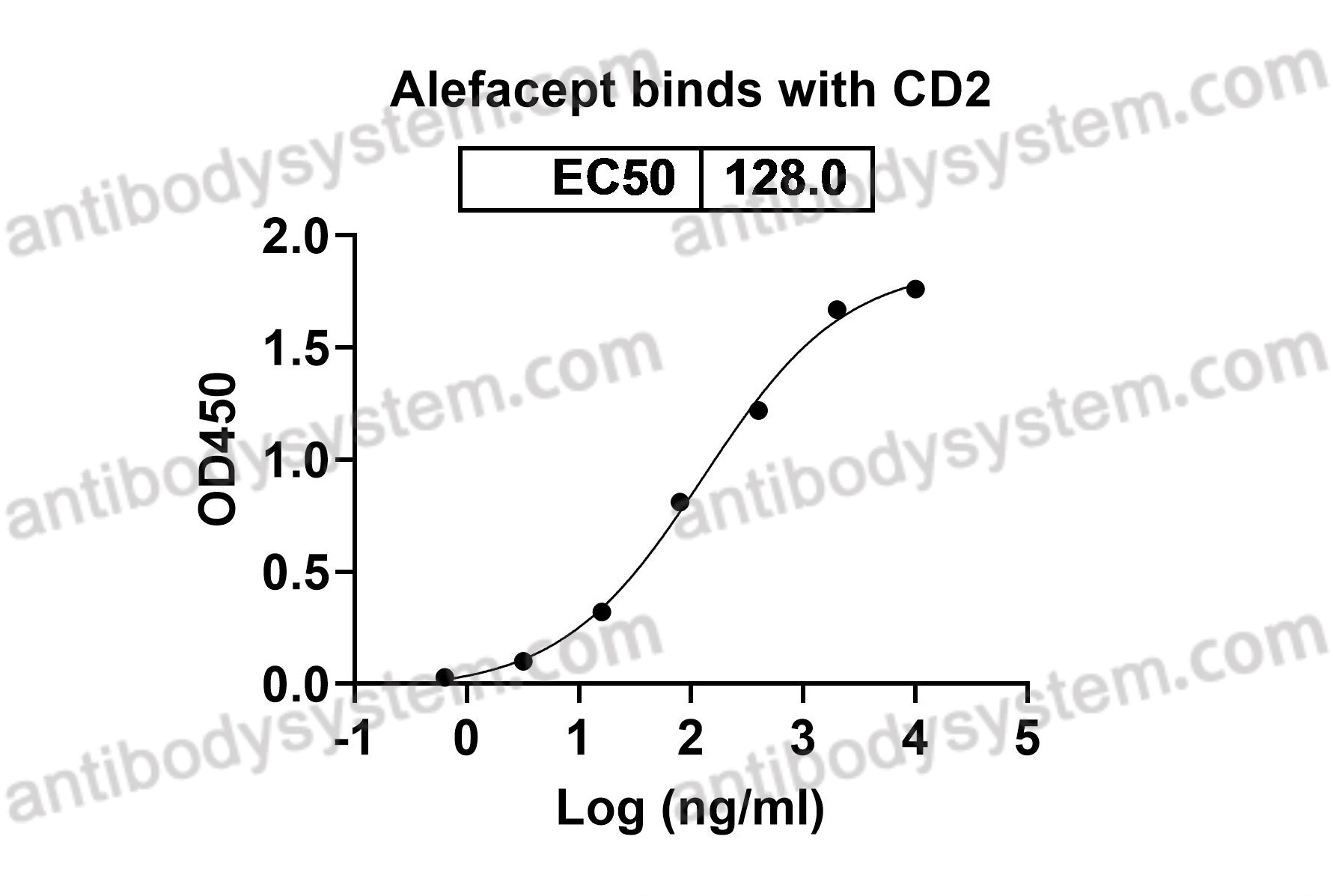
Fusion - [CD58 (LFA3, lymphocyte function associated antigen 3)]2 - IGHG1 Fc (Fragment constant)
Target
Erythrocyte receptor, SRBC, T-cell surface antigen CD2, Rosette receptor, CD2, LFA-3 receptor, T-cell surface antigen T11/Leu-5, LFA-2
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P06729
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CD58-Fc, LFA3-Fc, CAS: 222535-22-0
Clone ID
Alefacept
Immunosuppressive agents in diabetes treatment: Hope or despair?, PMID:40487626
Early expansion of TIGIT+PD1+ effector memory CD4 T cells via agonistic effect of alefacept in new-onset type 1 diabetes., PMID:40073269
The Dermatology Life Quality Index as the primary outcome in randomized clinical trials: a systematic review., PMID:38819233
Evolving Landscape of Biologic Therapy for Pediatric Psoriasis., PMID:38796269
A systematic review of case series and clinical trials investigating systemic oral or injectable therapies for the treatment of vitiligo., PMID:38454597
Islet-autoreactive CD4+ T cells are linked with response to alefacept in type 1 diabetes., PMID:37751304
Review of natural compounds for potential psoriasis treatment., PMID:36995575
Biologics Can Significantly Improve Dermatology Life Quality Index (DLQI) in Psoriatic Patients: A Systematic Review., PMID:35637943
Construction of miRNA-regulated drug-pathway network to screen drug repurposing candidates for multiple sclerosis., PMID:35356949
Cost-Effectiveness of Low-Dose Antithymocyte Globulin Versus Other Immunotherapies for Treatment of New-Onset Type 1 Diabetes., PMID:34704801
Risk of herpes zoster associated with biological therapies for psoriasis and psoriatic arthritis: A systematic review and meta-analysis., PMID:34622837
Risk of Herpes Zoster Among Psoriasis Patients Taking Biologics: A Network Meta-Analysis of Cohort Studies., PMID:34150802
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies., PMID:33807678
Exhausted-like CD8+ T cell phenotypes linked to C-peptide preservation in alefacept-treated T1D subjects., PMID:33351781
CD4+CD25+CD127hi cell frequency predicts disease progression in type 1 diabetes., PMID:33301420
Comparing Beta Cell Preservation Across Clinical Trials in Recent-Onset Type 1 Diabetes., PMID:32833543
CD2 Immunobiology., PMID:32582179
Structure-Based Design and Discovery of a Long-Acting Cocaine Hydrolase Mutant with Improved Binding Affinity to Neonatal Fc Receptor for Treatment of Cocaine Abuse., PMID:32189158
Evolution of the inclusion/exclusion criteria and primary endpoints in pivotal trials of biologics and small oral molecules for the treatment of psoriasis., PMID:32167790
In vitro Evidence That Combination Therapy With CD16-Bearing NK-92 Cells and FDA-Approved Alefacept Can Selectively Target the Latent HIV Reservoir in CD4+ CD2hi Memory T Cells., PMID:30455699
Systemic treatments for alopecia areata: A systematic review., PMID:30191561
ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors)., PMID:29427804
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis., PMID:29271481
Old and New Biological Therapies for Psoriasis., PMID:29104241
Anti‑cytokine therapy for psoriasis - not only TNF‑α blockers. Overview of reports on the effectiveness of therapy with IL‑12/IL‑23 and T and B lymphocyte inhibitors., PMID:28026823
Biological therapies (immunomodulatory drugs), worsening of psoriasis and rebound effect: new evidence of similitude., PMID:27914574
Can restoring immune balance be the ultimate therapy for type 1 diabetes?, PMID:27181881
Graft Versus Host Disease After Liver Transplantation in Adults: A Case series, Review of Literature, and an Approach to Management., PMID:27495762
Correlation Among Hypoglycemia, Glycemic Variability, and C-Peptide Preservation After Alefacept Therapy in Patients with Type 1 Diabetes Mellitus: Analysis of Data from the Immune Tolerance Network T1DAL Trial., PMID:27209482
A novel therapeutic paradigm for patients with extensive alopecia areata., PMID:27164008
Can We Repurpose FDA-Approved Alefacept to Diminish the HIV Reservoir?, PMID:27110598
Tailored treatment options for patients with psoriatic arthritis and psoriasis: review of established and new biologic and small molecule therapies., PMID:26892034
Targeting memory T cells in type 1 diabetes., PMID:26370695
Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients., PMID:26193635
Use of Alefacept for Preconditioning in Multiply Transfused Pediatric Patients with Nonmalignant Diseases., PMID:26095669
Unusual case of B cell lymphoma after immunosuppressive treatment for psoriasis., PMID:25914782
Chimeric fusion proteins used for therapy: indications, mechanisms, and safety., PMID:25832756
Neutralizing BAFF/APRIL with atacicept prevents early DSA formation and AMR development in T cell depletion induced nonhuman primate AMR model., PMID:25675879
Disseminated mycobacterium avium complex as protein-losing enteropathy in a non-HIV patient., PMID:25672059
Network Meta-analysis of Treatments for Chronic Plaque Psoriasis in Canada., PMID:25348757
Biologic treatment in Sjögren's syndrome., PMID:25342375
Biologics in dermatology: an integrated review., PMID:25284845
Acitretin-induced alopecia areata: a case report., PMID:25198403
Induction immunosuppressive therapy in kidney transplantation., PMID:24635795
Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial., PMID:24622414
Restoring immune balance in type 1 diabetes., PMID:24622404
Novel immunosuppressive agents in kidney transplantation., PMID:24392311
Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection., PMID:24354871
Diabetes: Immunotherapy for T1DM--still not there yet., PMID:24189510