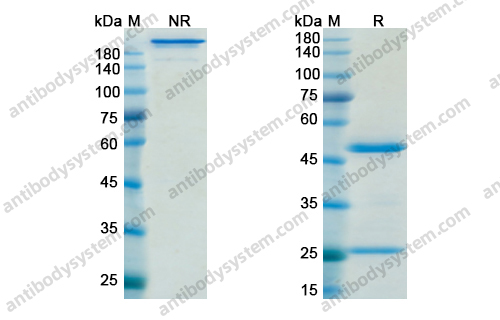
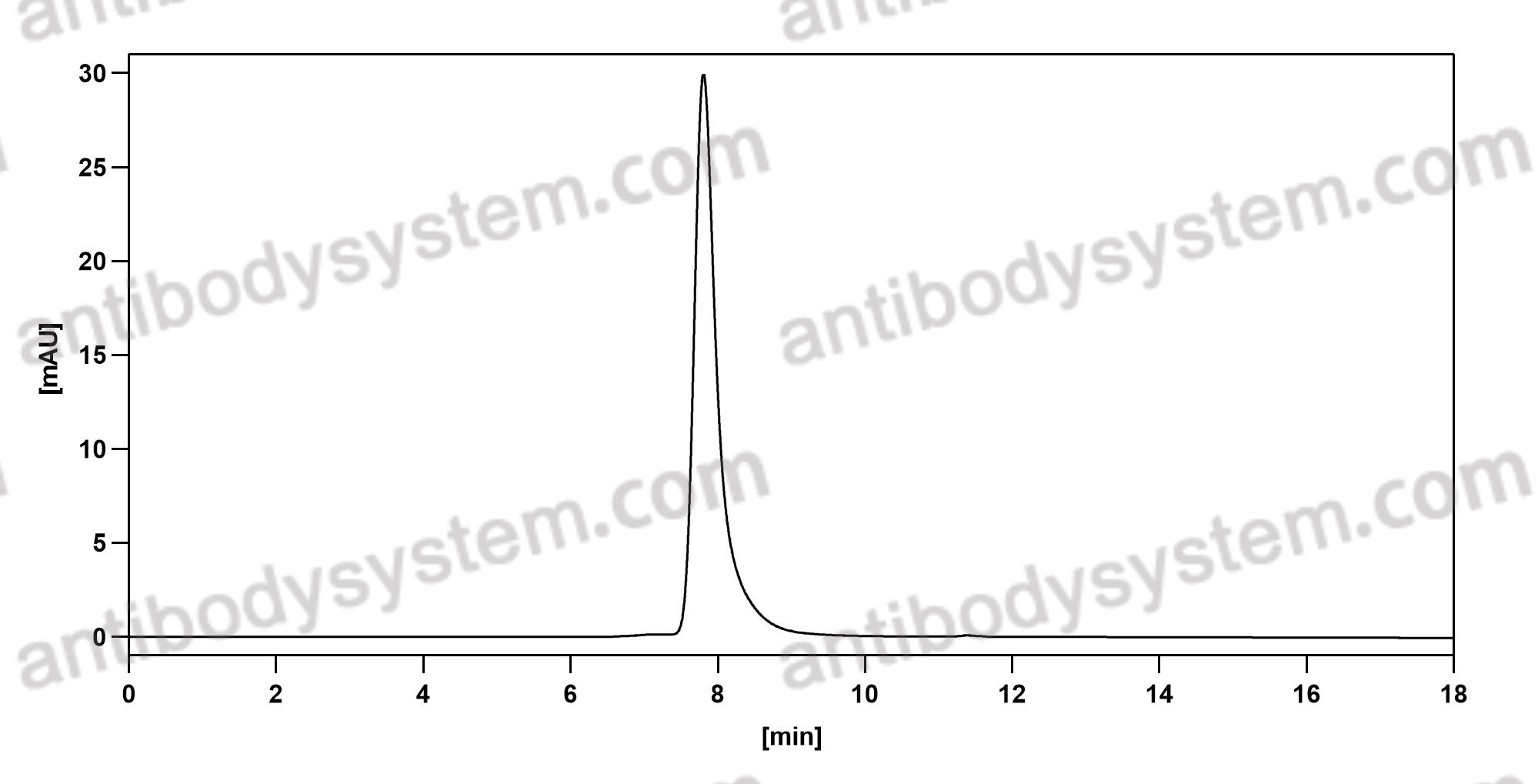
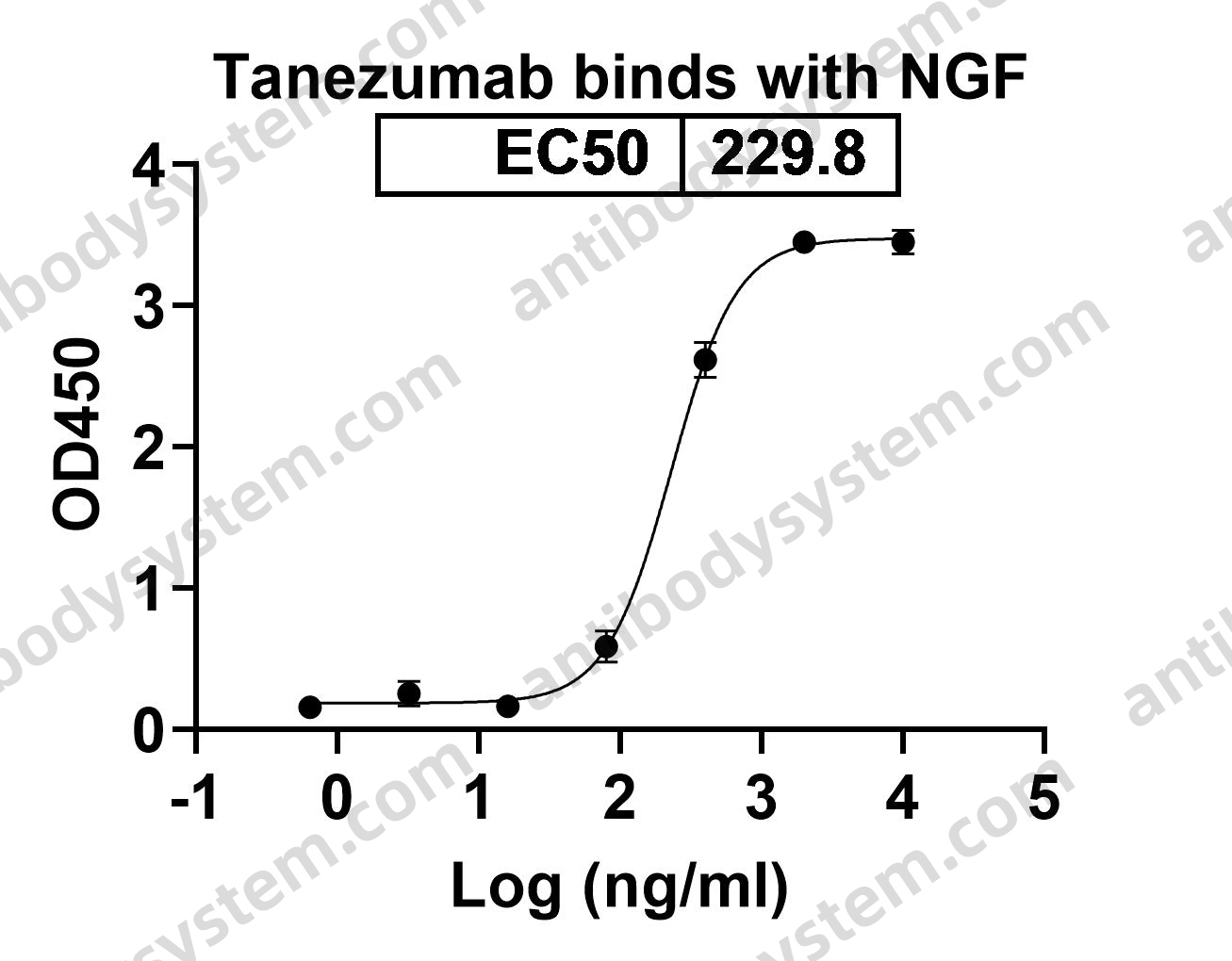
[Muromonab CD3], PMID: 15954439
[Muromonab CD3--the first monoclonal antibody used in humans], PMID: 7971509
Muromonab CD3. A review of its pharmacology and therapeutic potential, PMID: 2503348
Muromonab CD3: a reappraisal of its pharmacology and use as prophylaxis of solid organ transplant rejection, PMID: 8861551
Muromonab-CD3 and antithymocyte globulin in renal transplantation, PMID: 9391693
Muromonab-CD3, PMID: 1908673
Muromonab-CD3-induced neurotoxicity: report of two siblings, one of whom had subsequent cyclosporin-induced neurotoxicity, PMID: 11732768
Pharmacologic immunosuppression, PMID: 14766378
Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: bibliographic review, PMID: 8638282
Ten years of Orthoclone OKT3 (muromonab-CD3): a review, PMID: 9188368
Muromonab-CD3 therapy for refractory rejections after liver transplantation: a single-center experience during two decades in Japan, PMID: 20458505
Platelet procoagulant activity induced in vivo by muromonab-CD3 infusion in uremic patients, PMID: 11755950
Induction immunosuppression, PMID: 16969280
OKT3 (muromonab-CD3) associated hepatitis in a kidney transplant recipient, PMID: 12131696
Muromonab-CD3 (Orthoclone OKT3), methylprednisolone and cyclosporine for acute graft-versus-host disease prophylaxis in allogeneic bone marrow transplantation, PMID: 16862164
Muromonab CD3 (Orthoclone OKT3), PMID: 8531248
Aseptic meningitis and muromonab-CD3 therapy, PMID: 1905440
Antibody-enabled small-molecule drug discovery, PMID: 22743979
Muromonab-CD3 for the successful treatment of early chronic rejection after pediatric liver transplantation: report of a case, PMID: 21431500
Anti-CD3 antibodies, PMID: 8461387
Anti-CD3 antibody MacroGenics Inc, PMID: 16625825
A randomized multicenter comparison of basiliximab and muromonab (OKT3) in heart transplantation: SIMCOR study, PMID: 16770243
[Muromonab CD3 (Orthoclone OKT3) for the prophylaxis of heart allograft rejection. Hemodynamics and respiratory tolerance], PMID: 8572388
Engineered CD3 antibodies for immunosuppression, PMID: 12930354
Anaphylactoid reaction to muromonab-CD3 in a pediatric renal transplant recipient, PMID: 10641983
Remission of psoriatic lesions with muromonab-CD3 (orthoclone OKT3) treatment, PMID: 2502568
Falsely elevated plasma parathyroid hormone level mimicking tertiary hyperparathyroidism, PMID: 21247852
A pharmacoeconomic comparison of antithymocyte globulin and muromonab CD3 induction therapy in renal transplant recipients, PMID: 10165313
Teplizumab therapy for type 1 diabetes, PMID: 20095914
Reversible sensorineural hearing loss after renal transplant immunosuppression with OKT3 (muromonab-CD3), PMID: 9270425
Comparative study of muromonab-CD3 (OKT3) versus daclizumab (Zenapax) in cardiac transplantation at our center, PMID: 15866669
Induction therapy in cardiac transplantation: when and why?, PMID: 17545007
Generalized seizures associated with the use of muromonab-CD3 in two patients after kidney transplantation, PMID: 9161654
Immunosuppressive drugs, PMID: 1282440
Immunosuppressive preconditioning or induction regimens : evidence to date, PMID: 16956302
Monoclonal and polyclonal antibodies: clinical aspects, PMID: 1916908
CD3-specific antibody-induced active tolerance: from bench to bedside, PMID: 12563296
[Immunosuppressive therapy in renal transplantation], PMID: 8411796
Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion, PMID: 19348913
Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release, PMID: 23701319
Canadian Cardiovascular Society Consensus Conference update on cardiac transplantation 2008: Executive Summary, PMID: 19340342
Chemically Crosslinked Bispecific Antibodies for Cancer Therapy: Breaking from the Structural Restrictions of the Genetic Fusion Approach, PMID: 31973200
Comment: muromonab CD3 aseptic meningitis, PMID: 2508335
Mechanisms of action and overview of OKT3, PMID: 8588230
Generation and function of human regulatory CD8+ T cells induced by a humanized OKT3 monoclonal antibody hOKT3gamma1 (Ala-Ala), PMID: 18817833
Resetting the functional capacity of regulatory T cells: a novel immunotherapeutic strategy to promote immune tolerance, PMID: 16187942
Monoclonal antibodies in renal transplantation: a review, PMID: 1418316
Prophylactic use of OKT3 in liver transplantation. A review, PMID: 1914765
Humanized antibodies: enhancing therapeutic utility through antibody engineering, PMID: 8360588
Incidence and risk factors for skin cancer after heart transplantation: a systematic review and meta-analysis., PMID:39812815
Regulatory considerations in the design, development and quality of monoclonal antibodies and related products for the diagnosis and treatment of cancer., PMID:38746685
Myeloid and dendritic cells enhance therapeutics-induced cytokine release syndrome features in humanized BRGSF-HIS preclinical model., PMID:38384461
The future is on the way: β cell function preservation for type 1 diabetes., PMID:38218175
Teplizumab in Type 1 Diabetes Mellitus: An Updated Review., PMID:38187075
EGF-Receptor against Amphiregulin (AREG) Influences Costimulatory Molecules on Monocytes and T Cells and Modulates T-Cell Responses., PMID:38046264
Identification of potential therapeutic drugs targeting core genes for systemic lupus erythematosus (SLE) and coexisting COVID-19: Insights from bioinformatic analyses., PMID:38018597
Ex-vivo CS1-OKT3 dual specific bivalent antibody-armed effector T cells mediate cellular immunity against multiple myeloma., PMID:38012196
A multi-functional drug delivery nanosystem release of TLR-7 immunostimulant and OKT3 induced efficient cancer immunotherapy., PMID:37802276
Application of Computational Techniques in Antibody Fc-Fused Molecule Design for Therapeutics., PMID:37742298
Anti-CD79b/CD3 bispecific antibody combined with CAR19-T cells for B-cell lymphoma treatment., PMID:37707586
Novel anti-CD30/CD3 bispecific antibodies activate human T cells and mediate potent anti-tumor activity., PMID:37646042
Genetic code expansion in E. coli enables production of a functional 'ready-to-click' T cell receptor-specific scFv., PMID:37257818
Structure-based engineering of a novel CD3ε-targeting antibody for reduced polyreactivity., PMID:36991534
Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects., PMID:36505477
Development of therapeutic antibodies for the treatment of diseases., PMID:36418786
Risk-Based Control Strategies of Recombinant Monoclonal Antibody Charge Variants., PMID:36412839
Acute exercise mobilizes NKT-like cells with a cytotoxic transcriptomic profile but does not augment the potency of cytokine-induced killer (CIK) cells., PMID:36189306
Recent Progress in the Discovery and Development of Monoclonal Antibodies against Viral Infections., PMID:36009408
Survivin; a novel therapeutic target that correlates with survival of autoreactive T lymphocytes obtained from patients with ankylosing spondylitis., PMID:35995118
Optimizing rAAV6 transduction of primary T cells for the generation of anti-CD19 AAV-CAR-T cells., PMID:35658223
Development of a flow cytometry-based potency assay for prediction of cytokine storms induced by biosimilar monoclonal antibodies., PMID:35122772
SIRPγ-CD47 Interaction Positively Regulates the Activation of Human T Cells in Situation of Chronic Stimulation., PMID:34925315
Monoclonal Antibodies for Protozoan Infections: A Future Reality or a Utopic Idea?, PMID:34712683
Versatile and rapid microfluidics-assisted antibody discovery., PMID:34586015
Phosphatidylserine-Targeting Monoclonal Antibodies Exhibit Distinct Biochemical and Cellular Effects on Anti-CD3/CD28-Stimulated T Cell IFN-γ and TNF-α Production., PMID:34215655
Drug pipeline 1Q21-the old and the new., PMID:33981077
Peripheral T-cell lymphoma immunophenotype in a patient with a history of Muromonab-CD3 therapy: A case report and a diagnostic dilemma., PMID:33955673
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies., PMID:33807678
Development of the first reference antibody panel for qualification and validation of cytokine release assay platforms - Report of an international collaborative study., PMID:33458650
Nanovesicles released by OKT3 hybridoma express fully active antibodies., PMID:33404266
Developing an Arrayed CRISPR-Cas9 Co-Culture Screen for Immuno-Oncology Target ID., PMID:32375580
The FcγRIIIA-158 VV genotype increased the risk of post-transplant lymphoproliferative disorder in T-cell-depleted kidney transplant recipients - a retrospective study., PMID:32314433
Chemically Crosslinked Bispecific Antibodies for Cancer Therapy: Breaking from the Structural Restrictions of the Genetic Fusion Approach., PMID:31973200
Variable Benefits of Antibody Induction by Kidney Allograft Type., PMID:31865161
Stimulation of Mononuclear Cells Through Toll-Like Receptor 9 Induces Release of Microvesicles Expressing Double-Stranded DNA and Galectin 3-Binding Protein in an Interferon-α-Dependent Manner., PMID:31681284
Homotaurine Treatment Enhances CD4+ and CD8+ Regulatory T Cell Responses and Synergizes with Low-Dose Anti-CD3 to Enhance Diabetes Remission in Type 1 Diabetic Mice., PMID:31636084
Association of medication non-adherence with short-term allograft loss after the treatment of severe acute kidney transplant rejection., PMID:31623566
γδ T Cell-Secreted XCL1 Mediates Anti-CD3-Induced Oral Tolerance., PMID:31578268
Early suppression of peripheral mononuclear blood cells in sepsis in response to stimulation with cytomegalovirus, OKT3, and pokeweed mitogen., PMID:31545153
Immunologic Alterations Associated With Oral Delivery of Anti-CD3 (OKT3) Monoclonal Antibodies in Patients With Moderate-to-Severe Ulcerative Colitis., PMID:31423487
Bone marrow-liver-thymus (BLT) immune humanized mice as a model to predict cytokine release syndrome., PMID:31082370
Mature neutrophils suppress T cell immunity in ovarian cancer microenvironment., PMID:30730851
Immunosuppressive Total Nodal Irradiation-Based Reconditioning Regimens After Graft Rejection or Graft Failure in Pediatric Patients Treated With Myeloablative Allogeneic Hematopoietic Cell Transplantation., PMID:30593907
Pharmacokinetics and Immune Reconstitution Following Discontinuation of Thiopurine Analogues: Implications for Drug Withdrawal Strategies., PMID:30169593
Frequency of Human Papilloma Virus Occurrence Among Pathological Changes of the Oral Cavity in Kidney Allotransplant Recipients Undergoing Long-Term Pharmacological Immunosuppressive Therapy., PMID:30056920
TCRαβ/CD3 disruption enables CD3-specific antileukemic T cell immunotherapy., PMID:29997304
Sirolimus for Recurrent Giant Cell Myocarditis After Heart Transplantation: A Unique Therapeutic Strategy., PMID:29889677
Bispecific Nanosystems Enable Multieffector Immune Cell Retargeting for Hematologic Malignancy Therapy. [DHC27701]