Catalog No.
DVV02810
Expression system
Mammalian Cells
Species reactivity
Human respiratory syncytial virus A (strain A2)
Host species
Human
Isotype
IgG1, kappa
Clonality
Monoclonal
Target
F, Fusion glycoprotein F0, Fusion glycoprotein F2, p27, Intervening segment, Pep27, Peptide 27, Fusion glycoprotein F1
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
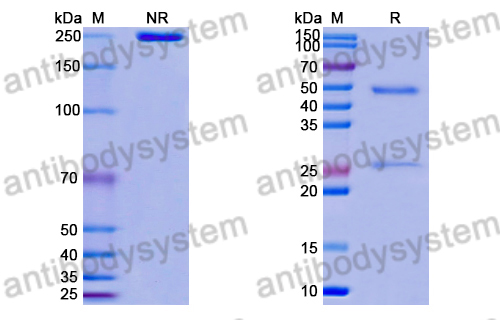
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P03420
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
ADI-19425
Clone ID
ADI-19425
Intention to Use RSVpreF Vaccine or Nirsevimab to Prevent Infant RSV Among Pregnant Individuals., PMID:40472256
Respiratory syncytial virus prophylaxis for children in Africa: Challenges, opportunities and public health strategies., PMID:40469396
Plasmonic optical fiber biosensors for ultra-low detection of respiratory syncytial virus via point-of-care tests., PMID:40467662
Fc-effector functional antibody assays for SARS-CoV-2 variants of concern., PMID:40463385
The potential public health impact of new immunization strategies for the prevention of RSV in children in Panama., PMID:40458968
Hydrophobic residue substitutions enhance the stability and in vivo immunogenicity of respiratory syncytial virus fusion protein., PMID:40434102
Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models., PMID:40432136
Immunogenicity of RSV Fusion Protein Adsorbed to Non-Pathogenic Bacillus subtilis Spores: Implications for Mucosal Vaccine Delivery in Nonclinical Animal Models., PMID:40426939
Respiratory syncytial virus: health burden, disease prevention, and treatment-recent progress and lessons learned., PMID:40420998
Clinical Insights and Advancements in Human Metapneumovirus Management and Prognosis., PMID:40351504
Synthetic Bacterial Membrane Vesicles as Versatile Antigen-Display Platforms against Respiratory Syncytial Virus., PMID:40343468
Immunogenicity evaluation of respiratory syncytial virus prefusogenic-F based virus-like-particles consisting of G and M proteins in mice., PMID:40334534
Cost-Effectiveness Analysis of Nirsevimab for the Prevention of Respiratory Syncytial Virus among Italian Infants., PMID:40317387
Editorial: Surveillance of Seasonal Respiratory Syncytial Virus (RSV) Infection in Children and Vulnerable Adults Drives Vaccine Development and New Immunization Programs., PMID:40308086
Nirsevimab and Maternal Respiratory Syncytial Virus Vaccine Recommendations for the Pediatric Population., PMID:40305635
A single amino acid mutation alters multiple neutralization epitopes in the respiratory syncytial virus fusion glycoprotein., PMID:40295799
Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods., PMID:40266209
Immunogenicity, safety, and tolerability of a β-glucan-CpG-adjuvanted respiratory syncytial virus vaccine in Japanese healthy participants aged 60 to 80 years: A phase 2, randomized, double-blind, dose-finding study., PMID:40257186
Summary of the National Advisory Committee on Immunization (NACI) Statement on the Prevention of Respiratory Syncytial Virus (RSV) in Infants., PMID:40241712
Functional implications of respiratory syncytial virus F sequence variability: a comparative analysis using contemporary RSV isolates., PMID:40227055
Development and evaluation of ELISA serological immunoassays for influenza and respiratory syncytial viruses., PMID:40215609
Recent advances in the prevention and treatment of respiratory syncytial virus disease., PMID:40202895
A bivalent mRNA vaccine against RSV infection in rodent models., PMID:40196112
Evaluation of dual pathogen recognition receptor agonists as adjuvants for respiratory syncytial virus - virus-like particles for pulmonary delivery., PMID:40176816
Strength and durability of RSV pre-fusion F IgG following infection and exposure in a household cohort, 2014-2022., PMID:40176487
Respiratory syncytial virus prefusion F3 vaccine in lung transplant recipients elicits CD4+ T cell response in all vaccinees., PMID:40169094
RSV F evolution escapes some monoclonal antibodies but does not strongly erode neutralization by human polyclonal sera., PMID:40161760
Intranasal Inoculation of Cationic Crosslinked Carbon Dots-Adjuvanted Respiratory Syncytial Virus F Subunit Vaccine Elicits Mucosal and Systemic Humoral and Cellular Immunity., PMID:40135196
Generation of nanobodies against the F protein of respiratory syncytial virus and establishment of an indirect immunofluorescence assay., PMID:40130866
Layer-by-Layer Microparticle Vaccines Containing a S177Q Point Mutation in the Central Conserved Domain of the RSV G Protein Improves Immunogenicity., PMID:40126409
Safety and efficacy of long-term gantenerumab treatment in dominantly inherited Alzheimer's disease: an open-label extension of the phase 2/3 multicentre, randomised, double-blind, placebo-controlled platform DIAN-TU trial., PMID:40120616
Recovery of Antibody Immunity After a Resurgence of Respiratory Syncytial Virus Infections., PMID:40099894
Binding and neutralising antibodies to respiratory syncytial virus and influenza A virus in serum and bronchoalveolar lavage fluid of healthy adults in the United States: A cross-sectional study., PMID:40037127
Development, Current Status, and Remaining Challenges for Respiratory Syncytial Virus Vaccines., PMID:40006644
Safety and efficacy of long-term gantenerumab treatment in dominantly inherited Alzheimer's disease: an open label extension of the phase 2/3 multicentre, randomised, double-blind, placebo-controlled platform DIAN-TU Trial., PMID:39974075
Respiratory Syncytial Virus Vaccine and Nirsevimab Uptake Among Pregnant People and Their Neonates., PMID:39969879
Epitope-focused vaccine immunogens design using tailored horseshoe-shaped scaffold., PMID:39966941
Enhanced construction of a bivalent adenovirus-based vaccine for preventing childhood pathogens: Human respiratory syncytial virus and norovirus., PMID:39952326
A truncated pre-F protein mRNA vaccine elicits an enhanced immune response and protection against respiratory syncytial virus., PMID:39910047
Molecular characterization of RSV infections in elderly patients during the 2023/2024 season in the era of nirsevimab introduction., PMID:39895281
Efficacy, immunogenicity, and safety of an investigational maternal respiratory syncytial virus prefusion F protein-based vaccine., PMID:39879629
Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus., PMID:39852872
An mRNA-Based Respiratory Syncytial Virus Vaccine Elicits Strong Neutralizing Antibody Responses and Protects Rodents Without Vaccine-Associated Enhanced Respiratory Disease., PMID:39852831
Anti-Idiotypic Antibody as a Booster Vaccine Against Respiratory Syncytial Virus., PMID:39852814
Protective or limited? Maternal antibodies and RSV-associated lower respiratory tract infection in hospitalized infants aged 28-90 days., PMID:39845974
The recent landscape of RSV vaccine research., PMID:39802673
Evaluation of an F Protein-Based Recombinant Protein for Immunization Against Respiratory Syncytial Virus., PMID:39791530
Fumarprotocetraric acid and geraniin were identified as novel inhibitors of human respiratory syncytial virus infection in vitro., PMID:39776441
Evaluating the Impact of N-Glycan Sequon Removal in the p27 Peptide on RSV F Protein Immunogenicity and Functionality., PMID:39772158
A Statistical Model to Predict Protection Against Infant Respiratory Syncytial Virus Disease Through Maternal Immunization., PMID:39772012