Catalog No.
KDJ04002
Description
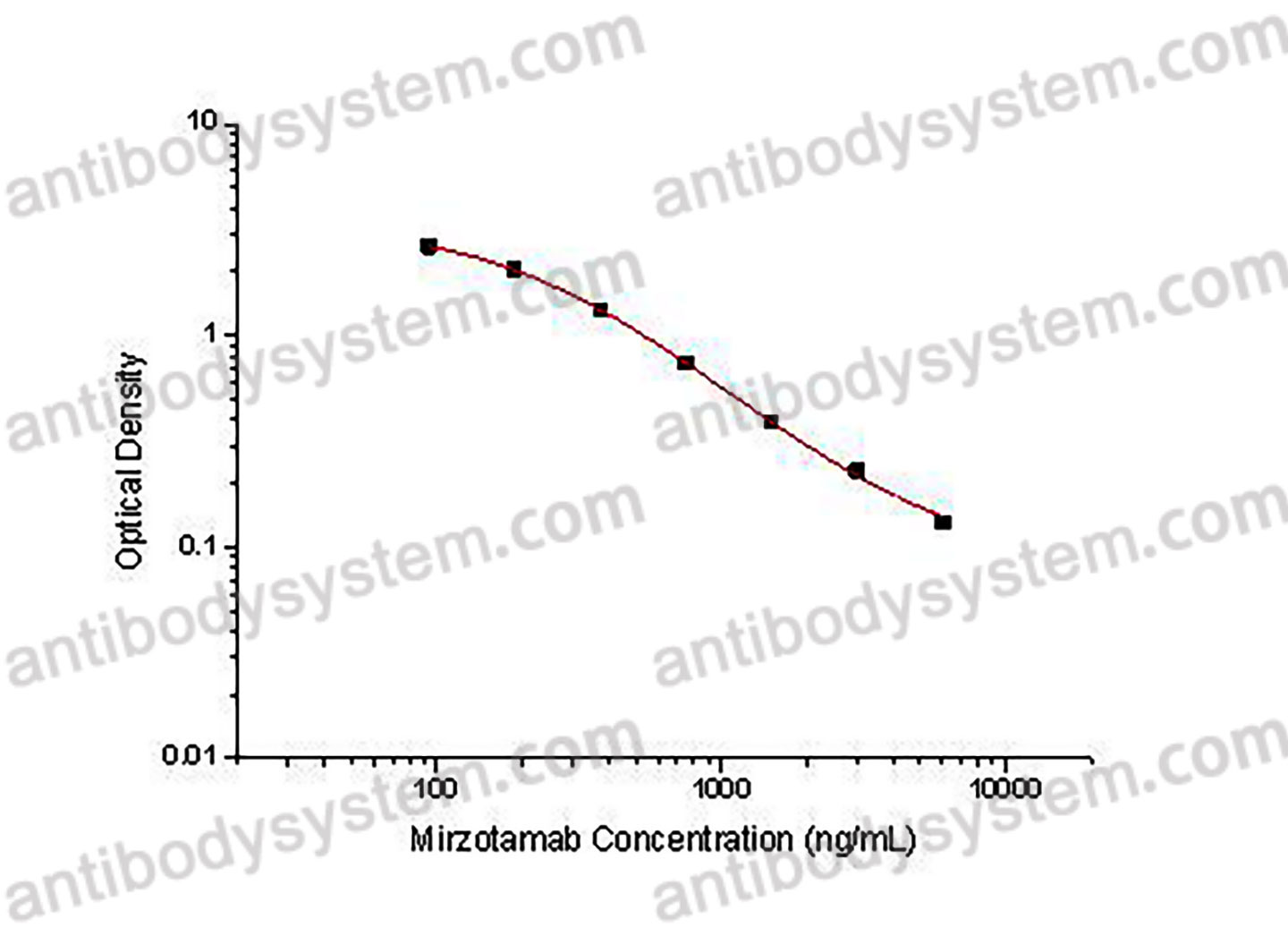
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD276 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Mirzotamab in the sample competitively binds to the pre-coated protein with biotin-labeled Mirzotamab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Mirzotamab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Mirzotamab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
93.75 - 6,000 ng/mL
Sensitivity
45.23 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
3805.7 |
871.1 |
349.7 |
3885.1 |
1031.6 |
409.2 |
|
Standard deviation |
233.0 |
89.1 |
39.3 |
509.5 |
109.4 |
56.5 |
|
CV (%) |
6.1 |
10.2 |
11.2 |
13.1 |
10.6 |
13.8 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ABBV-155, CAS: 2229859-11-2
Background
Mirzotamab clezutoclax (ABBV-155) is a first-in-class antibody drug conjugate comprised of a BCL-X L (B-cell lymphoma - extra long) inhibitor, solubilizing linker, and a monoclonal anti-B7H3 antibody. Methods: Patients (pts) with relapsed and/or refractory (R/R) solid tumors were administered mirzotamab clezutoclax with or without paclitaxel. Dose escalation of mirzotamab clezutoclax was guided by Bayesian continual reassessment.