Catalog No.
KDG52001
Description
PRINCIPLE OF THE ASSAY
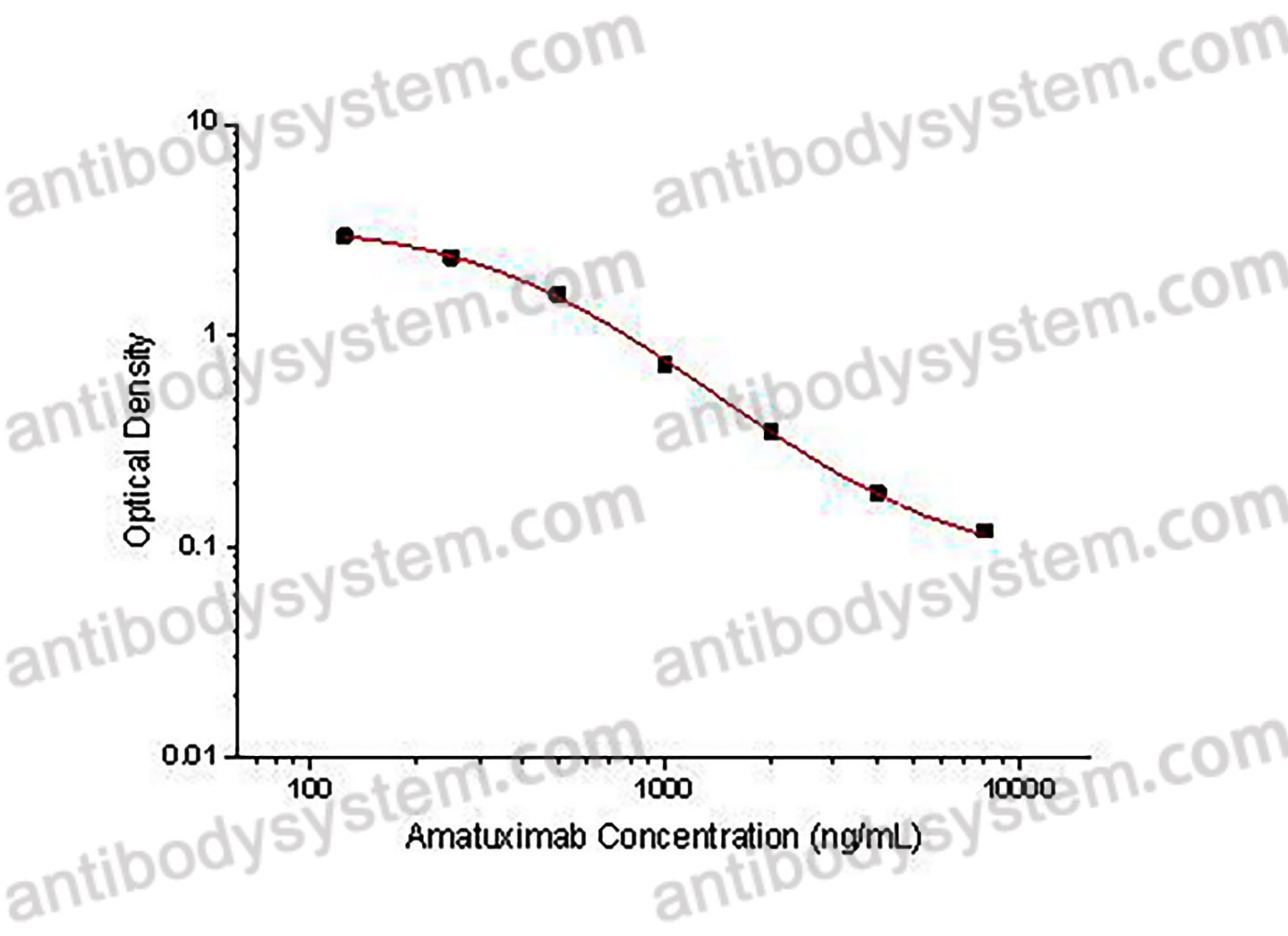
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human MSLN has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Amatuximab in the sample competitively binds to the pre-coated protein with biotin-labeled Amatuximab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Amatuximab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Amatuximab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
125 - 8,000 ng/mL
Sensitivity
78.43 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
4417.2
|
1223.4
|
257.1
|
4619.2
|
1151.9
|
250.2
|
|
Standard deviation
|
189.4
|
154.2
|
8.9
|
413.3
|
88.0
|
33.2
|
|
CV (%)
|
4.3
|
12.6
|
3.5
|
8.9
|
7.6
|
13.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
MORAb-009, CAS: 931402-35-6
Background
Amatuximab, alternative names: MORAb-009, anti-MSLN monoclonal antibody (MAb) is a chimeric, humanized IgG1/k MAb that targets the cell surface Mesothelin (MSLN). The precursor of MORAb-009 was isolated by Chowdhury et al in 1998 56 from a mouse splenic mRNA and was then optimized by fusing the gene encoding MSLN Fv (SS1 scFv) with human IgG1 and kappa regions. It was first designed to treat patients with mesothelin-positive cancers (pancreatic, ovarian, mesothelioma and lung). Amatuximab is not yet commercially available, and the highest development phase is Phase II. In addition, on 16 January 2014, orphan designation (EU/3/13/1222) was granted by the European Commission to Eisai Europe Limited, United Kingdom, for amatuximab for the treatment of malignant mesothelioma.
Preclinical evaluation of 89Zr/177Lu-labeled amatuximab for theranostic application in pancreatic ductal adenocarcinoma., PMID:39542119
Mesothelin antigen density influences anti-mesothelin chimeric antigen receptor T cell cytotoxicity., PMID:38349311
Rapid nanobody-based imaging of mesothelin expressing malignancies compatible with blocking therapeutic antibodies., PMID:37388728
Immunotherapeutic Targeting of Mesothelin Positive Pediatric AML Using Bispecific T Cell Engaging Antibodies., PMID:34885074
Early administration of amatuximab, a chimeric high-affinity anti-mesothelin monoclonal antibody, suppresses liver metastasis of mesothelin-expressing pancreatic cancer cells and enhances gemcitabine sensitivity in a xenograft mouse model., PMID:33905019
Mesothelin blockage by Amatuximab suppresses cell invasiveness, enhances gemcitabine sensitivity and regulates cancer cell stemness in mesothelin-positive pancreatic cancer cells., PMID:33637083
The anti-mesothelin monoclonal antibody amatuximab enhances the anti-tumor effect of gemcitabine against mesothelin-high expressing pancreatic cancer cells in a peritoneal metastasis mouse model., PMID:30333914
Tumor-Shed Antigen Affects Antibody Tumor Targeting: Comparison of Two 89Zr-Labeled Antibodies Directed against Shed or Nonshed Antigens., PMID:29720923
CA125 suppresses amatuximab immune-effector function and elevated serum levels are associated with reduced clinical response in first line mesothelioma patients., PMID:29652548
[Systemic Treatment of Malignant Pleural Mesothelioma]., PMID:29361614
Amatuximab and novel agents targeting mesothelin for solid tumors., PMID:29184420
Mesothelin Immunotherapy for Cancer: Ready for Prime Time?, PMID:27863199
Novel Antibody Therapeutics Targeting Mesothelin In Solid Tumors., PMID:27853672
Possible new therapeutic agents for malignant pleural mesothelioma: anti-CD26 monoclonal antibody and naftopidil., PMID:27718761
Tackling Mesothelioma With Immunotherapies., PMID:27510518
Population pharmacokinetics and exposure-response relationship of amatuximab, an anti-mesothelin monoclonal antibody, in patients with malignant pleural mesothelioma and its application in dose selection., PMID:26898299
Tumor and organ uptake of (64)Cu-labeled MORAb-009 (amatuximab), an anti-mesothelin antibody, by PET imaging and biodistribution studies., PMID:26307499
Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using single photon emission computed tomography-computed tomography (SPECT-CT) imaging., PMID:25756664
Phase I study of amatuximab, a novel monoclonal antibody to mesothelin, in Japanese patients with advanced solid tumors., PMID:25502863
Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma., PMID:25231400
Characterization of crystals of an antibody-recognition fragment of the cancer differentiation antigen mesothelin in complex with the therapeutic antibody MORAb-009., PMID:22869130
Recognition of mesothelin by the therapeutic antibody MORAb-009: structural and mechanistic insights., PMID:22787150
The role of mesothelin in tumor progression and targeted therapy., PMID:22721387
Effect of chelator conjugation level and injection dose on tumor and organ uptake of 111In-labeled MORAb-009, an anti-mesothelin antibody., PMID:21741258
Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers., PMID:21037025
Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin., PMID:18088084
Mesothelin targeted cancer immunotherapy., PMID:17945478