Catalog No.
KDG11002
Description
PRINCIPLE OF THE ASSAY
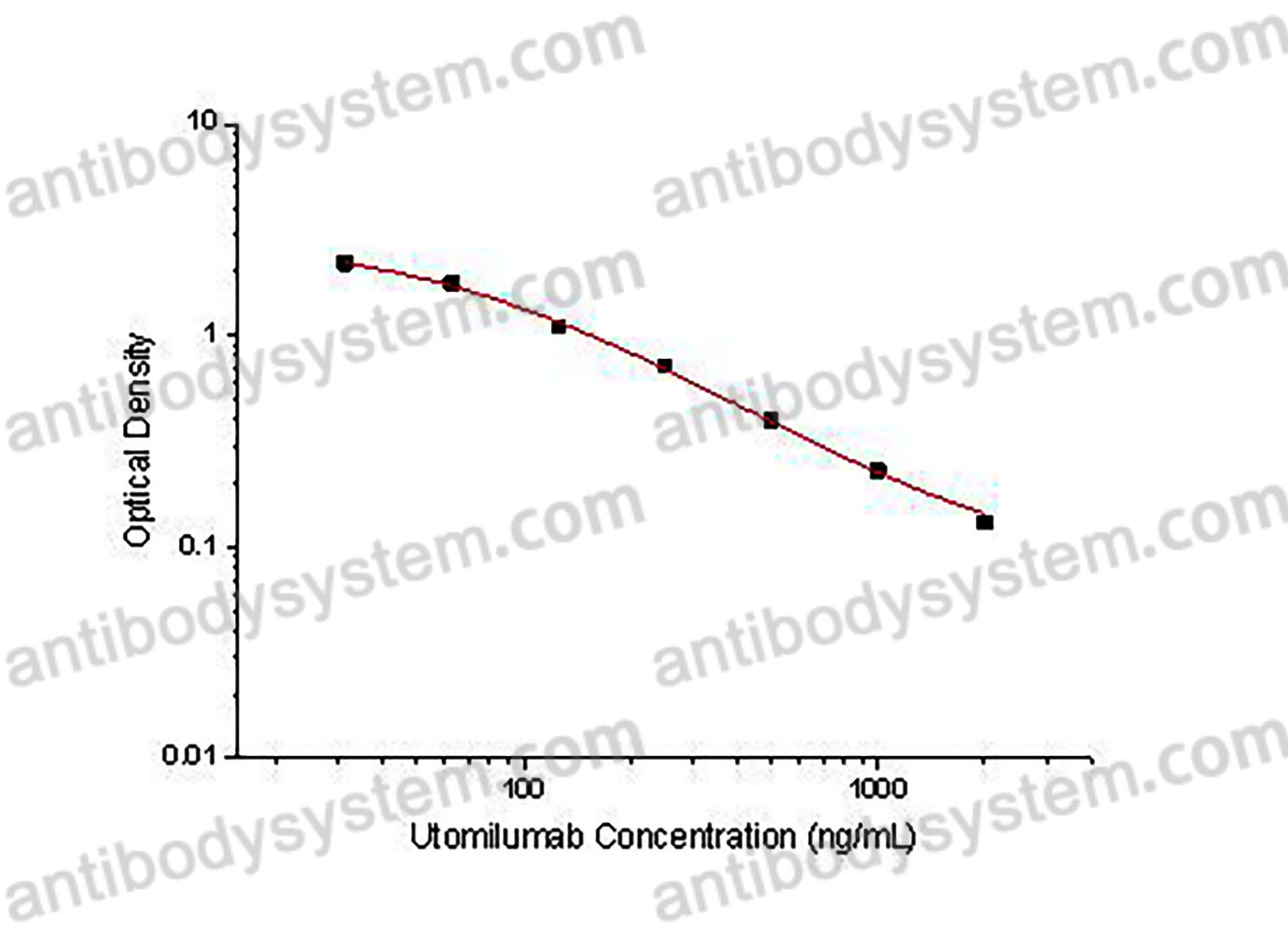
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD137 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Utomilumab in the sample competitively binds to the pre-coated protein with biotin-labeled Utomilumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Utomilumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Utomilumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
8.32 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
879.1
|
241.1
|
56.9
|
815.5
|
226.0
|
53.8
|
|
Standard deviation
|
49.9
|
16.5
|
6.0
|
82.7
|
18.2
|
6.7
|
|
CV (%)
|
5.7
|
6.8
|
10.6
|
10.1
|
8.1
|
12.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
PF-05082566, PF-2566, CAS: 1417318-27-4
Background
Utomilumab is the proposed non-proprietary name for PF-05082566, an investigational immunotherapy and a fully human IgG2 agonist monoclonal antibody (mAb) that binds to the extracellular domain of human 4-1BB/CD137 with high affinity and specificity. It is developed by Pfizer to treat advanced cancers either alone or in combination with other therapies. When utomilumab is injected into a person's bloodstream, it binds to 4-1BB on immune cells and stimulates them. At present, the approval of the monoclonal antibody has not been disclosed in the relevant authoritative drug approval agency, such as the European Medicines Agency and the U.S. Food and Drug Administration (FDA).
A phase I/II trial of avelumab combinations with ivuxolimab, utomilumab, and radiation therapy in patients with advanced gastrointestinal malignancies., PMID:40139261
Development of a c-MET x CD137 bispecific antibody for targeted immune agonism in cancer immunotherapy., PMID:38492435
Facile generation of biepitopic antibodies with intrinsic agonism for activating receptors in the tumor necrosis factor superfamily., PMID:38168220
M9657 Is a Bispecific Tumor-Targeted Anti-CD137 Agonist That Induces MSLN-Dependent Antitumor Immunity without Liver Inflammation., PMID:38091375
Phase 1/2 trial of avelumab combined with utomilumab (4-1BB agonist), PF-04518600 (OX40 agonist), or radiotherapy in patients with advanced gynecologic malignancies., PMID:37864520
A multi-cohort phase 1b trial of rituximab in combination with immunotherapy doublets in relapsed/refractory follicular lymphoma., PMID:37851072
Costimulatory capacity of CD137 mAbs on T cells depends on elaborate CRD structures but not on blocking ligand-receptor binding., PMID:37675596
FcγR requirements and costimulatory capacity of Urelumab, Utomilumab, and Varlilumab., PMID:37575254
Axicabtagene Ciloleucel in Combination with the 4-1BB Agonist Utomilumab in Patients with Relapsed/Refractory Large B-Cell Lymphoma: Phase 1 Results from ZUMA-11., PMID:37527011
4-1BB Targeting Immunotherapy: Mechanism, Antibodies, and Chimeric Antigen Receptor T., PMID:37433196
The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy., PMID:36727218
First-in-human study of an OX40 (ivuxolimab) and 4-1BB (utomilumab) agonistic antibody combination in patients with advanced solid tumors., PMID:36302562
Development and characterization of a novel human CD137 agonistic antibody with anti-tumor activity and a good safety profile in non-human primates., PMID:36176235
A humanized 4-1BB-targeting agonistic antibody exerts potent antitumor activity in colorectal cancer without systemic toxicity., PMID:36076251
Utomilumab in Patients With Immune Checkpoint Inhibitor-Refractory Melanoma and Non-Small-Cell Lung Cancer., PMID:35983060
Avelumab in Combination Regimens for Relapsed/Refractory DLBCL: Results from the Phase Ib JAVELIN DLBCL Study., PMID:34687398
CD137 as an Attractive T Cell Co-Stimulatory Target in the TNFRSF for Immuno-Oncology Drug Development., PMID:34064598
First-in-Human Study of Utomilumab, a 4-1BB/CD137 Agonist, in Combination with Rituximab in Patients with Follicular and Other CD20+ Non-Hodgkin Lymphomas., PMID:32144134
Epitope and Fc-Mediated Cross-linking, but Not High Affinity, Are Critical for Antitumor Activity of CD137 Agonist Antibody with Reduced Liver Toxicity., PMID:31974274
A phase Ib study of utomilumab (PF-05082566) in combination with mogamulizumab in patients with advanced solid tumors., PMID:31801624
Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity., PMID:31105267
Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab., PMID:30410017
Limited Cross-Linking of 4-1BB by 4-1BB Ligand and the Agonist Monoclonal Antibody Utomilumab., PMID:30355497
Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer., PMID:29549159
Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies., PMID:29118009
Combination avelumab and utomilumab immunotherapy can induce diabetic ketoacidosis., PMID:28648834
Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors., PMID:28634283
Enhancing PD-1 Blockade in Solid Tumors., PMID:27369047
Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity., PMID:22406983