Catalog No.
KDE20501
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative sandwich enzyme immunoassay technique. Recombinant Human ACVRL1 has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Ascrinvacumab present is bound by the immobilized antibody. After washing away any unbound substances, a biotin-labeled Human ACVRL1 is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Dupilumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
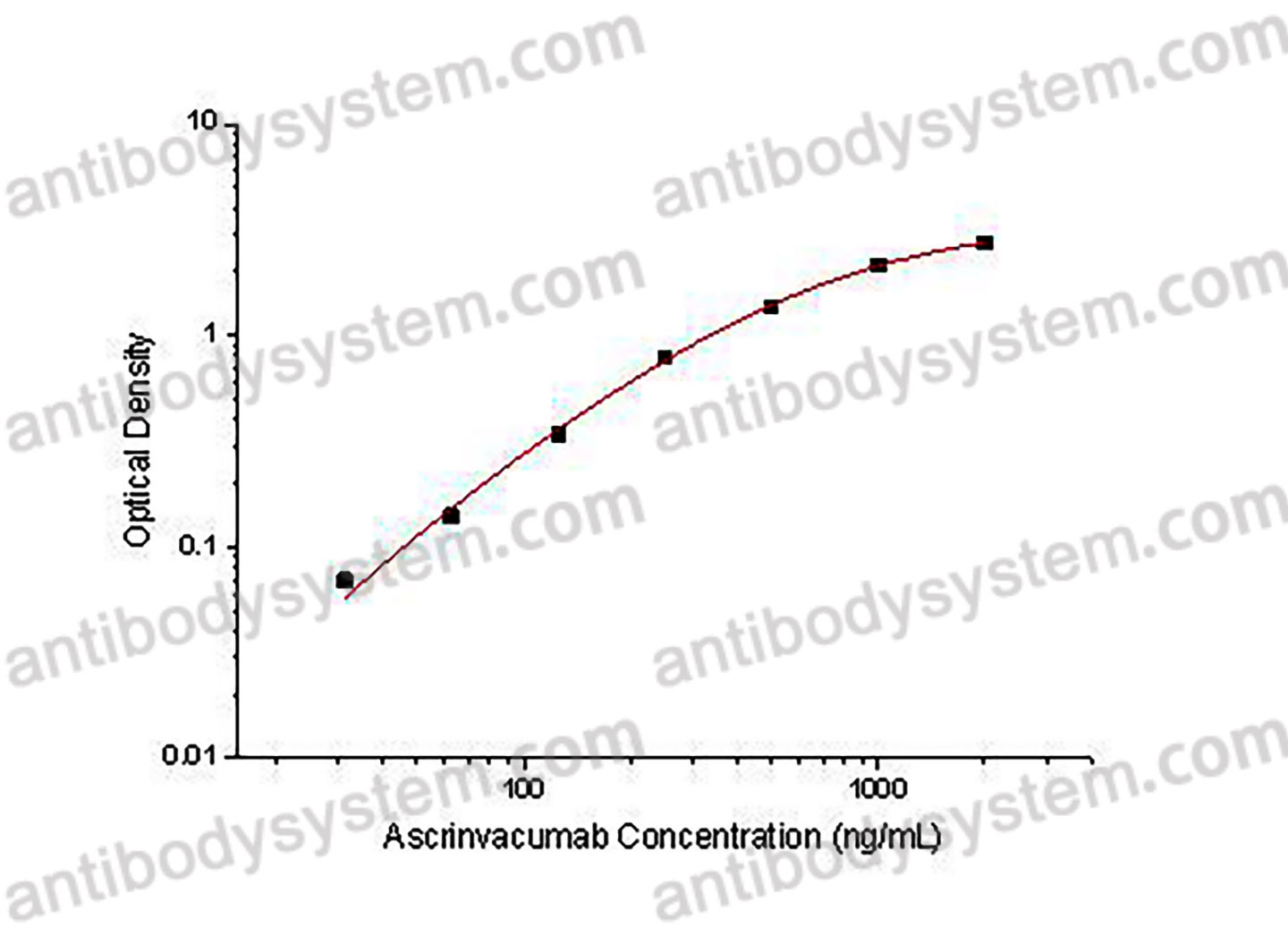
Used for the quantitative determination of Ascrinvacumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
17.65 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
933.4 |
198.6 |
43.7 |
859.8 |
205.5 |
57.5 |
|
Standard deviation |
50.2 |
9.0 |
3.4 |
63.2 |
10.1 |
5.3 |
|
CV (%) |
5.4 |
4.5 |
7.9 |
7.4 |
4.9 |
9.3 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
PF-03446962, , CAS: 1463459-96-2
Background
Ascrinvacumab, also known as PF-03446962 or anti-hALK1 antibody, is a human immunoglobulin G (IgG) 2 monoclonal antibody which can be used as therapeutic antibody drug for the treatment of many tumors. It has been found that the clinical trials of ascrinvacumab has been developed in cancers including advanced solid tumors, urothelial cell carcinoma, hepatocellular carcinoma, and mesothelioma. Ascrinvacumab can binding to ACVRL1 by recognizing the extracellular domain of ACVRL1. ACVRL1, also called activin receptor-like kinase 1 (ALK1), is a transforming growth factor β (TGF-β) type I receptor. Ascrinvacumab is a fully human monoclonal antibody generated by immunizing the IgG 2 transgenic XenoMouse. In a human/mouse chimera tumor model, ascrinvacumab decreased human vessel density and improved antitumor efficacy when combined with bevacizumab (anti-VEGF). It suggests that ascrinvacumab therapy may be complementary to anti-VEGF in cancer intervention. As early as 2012, researchers had investigated the effects of ascrinvacumab on endothelial cell function. And the results showed that ascrinvacumab do interferes with the endothelial cell sprouting induced by Bone Morphogenetic Protein 9 (BMP9). A phase 2 trial study of ascrinvacumab in pre-treated patients with urothelial cancer had been reported in 2014. In addition, its treatment results of advanced solid tumors, hepatocellular carcinoma, and advanced malignant pleural mesothelioma also had been reported in 2016. In addition to advanced malignant pleural mesothelioma, ascrinvacumab shows the value of continuing treatment evaluation in the other two diseases.