Catalog No.
KDD37501
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD33 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Lintuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Lintuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Lintuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Lintuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
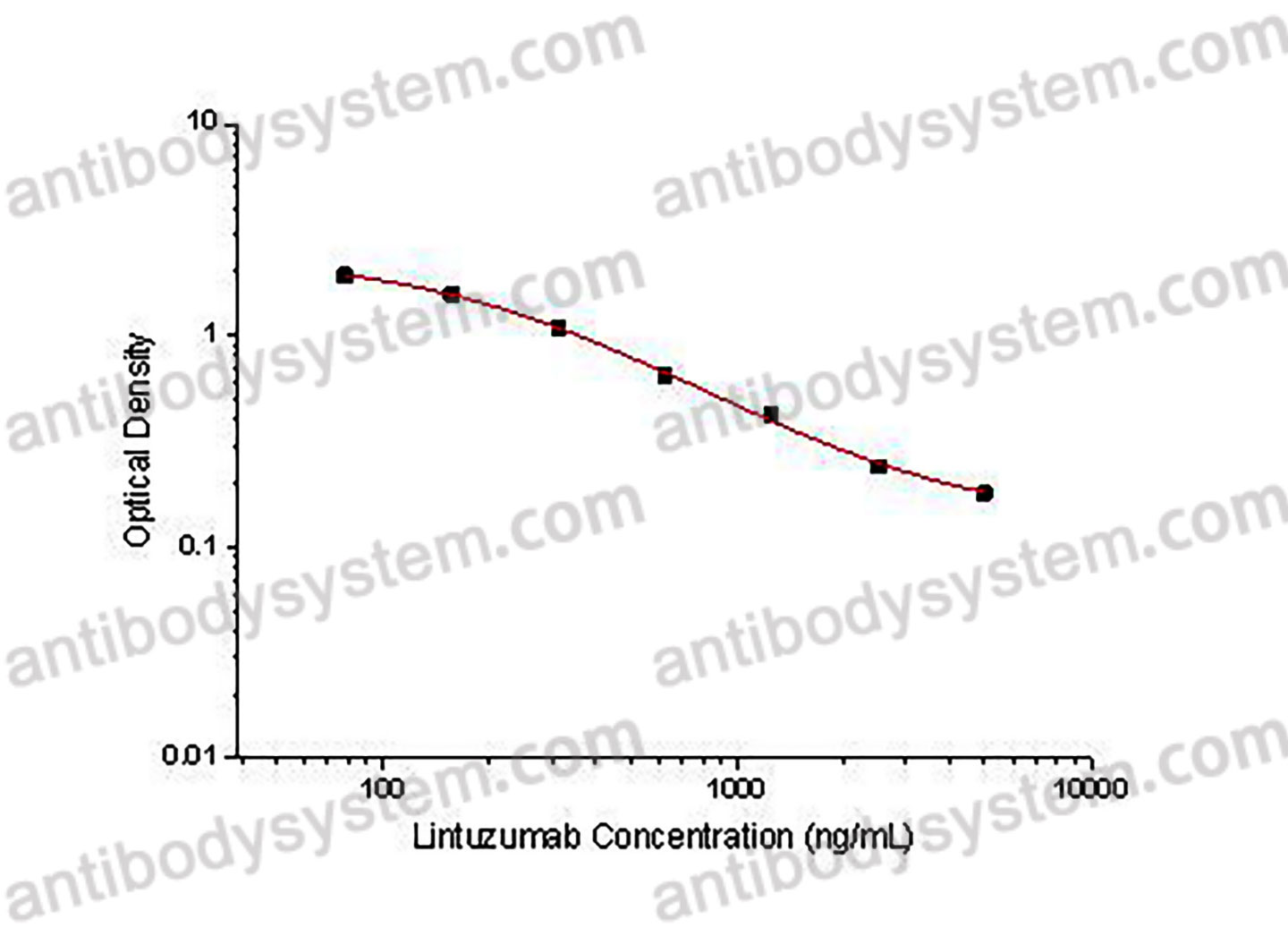
Range
78.13 - 5,000 ng/mL
Sensitivity
39.06 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2593.7
|
680.7
|
164.3
|
2490.9
|
699.3
|
253.3
|
|
Standard deviation
|
293.3
|
87.3
|
23.5
|
223.8
|
132.2
|
37.2
|
|
CV (%)
|
11.3
|
12.8
|
14.3
|
9.0
|
18.9
|
14.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
225Ac-lintuzumab, HuM195, SGN-33, SMART M195, HuM195, CAS: 166089-32-3
Background
Lintuzumab is a humanized monoclonal antibody, HuM195, that targets the cell surface antigen CD33 that is expressed on the vast majority of acute myeloid leukemia (AML) cells. [225Ac]Ac-lintuzumab clinical trials were discussed in detail in reference. An initial phase I dose-escalation trial demonstrated that for a single infusion of [225Ac]Ac-lintuzumab in patients with relapsed or refractory acute myeloid leukemia, the maximum tolerated dose (MTD) was determined to be 111 kBq/kg with antileukemic activity across all dose levels. No evidence of radiation-induced nephrotoxicity was seen. Peripheral blasts were eliminated in 63% of the patients at doses of >37 kBq/kg. Bone marrow blast reduction was observed in 67% of patients. Subsequently, a multicenter phase I dose-escalation trial was conducted to define MTD, toxicity, and response rate of fractionated-dose [225Ac]Ac-lintuzumab when combined with low-dose cytarabine (LDAC) in older patients with untreated AML who were not candidates for intensive chemotherapy.
Phase 1 study of lintuzumab-Ac225 combined with CLAG-M salvage therapy in relapsed/refractory acute myeloid leukemia., PMID:39955432
89Zr-immunoPET-guided selection of a CD33xIL15 fusion protein optimized for antitumor immune cell activation and in vivo tumour retention in acute myeloid leukaemia., PMID:38987489
Developing a membrane-proximal CD33-targeting CAR T cell., PMID:38772686
Targeting CD33+ Acute Myeloid Leukemia with GLK-33, a Lintuzumab-Auristatin Conjugate with a Wide Therapeutic Window., PMID:38561023
HuM195 and its single-chain variable fragment increase Aβ phagocytosis in microglia via elimination of CD33 inhibitory signaling., PMID:38383769
In Vitro and In Vivo Characterization of 89Zirconium-Labeled Lintuzumab Molecule., PMID:36235126
Where do we stand with radioimmunotherapy for acute myeloid leukemia?, PMID:35350938
Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab., PMID:35247915
Radiation Safety Considerations and Clinical Advantages of α-Emitting Therapy Radionuclides., PMID:34750237
Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design., PMID:34531250
225Ac-labeled CD33-targeting antibody reverses resistance to Bcl-2 inhibitor venetoclax in acute myeloid leukemia models., PMID:33347715
CD33-Targeted Therapies: Beating the Disease or Beaten to Death?, PMID:32875599
Targeted Alpha-Particle Therapy for Hematologic Malignancies., PMID:32172800
CD33 splice site genotype was not associated with outcomes of patients receiving the anti-CD33 drug conjugate SGN-CD33A., PMID:31439003
Targeted Alpha-Particle Therapy for Hematologic Malignancies., PMID:31253514
Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies., PMID:29793418
Investigational CD33-targeted therapeutics for acute myeloid leukemia., PMID:29534618
Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors., PMID:29206680
Advances in immunotherapy for pediatric acute myeloid leukemia., PMID:28945115
Harnessing the Immune System Against Leukemia: Monoclonal Antibodies and Checkpoint Strategies for AML., PMID:28321813
In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia., PMID:27535972
ADCs Show Promise in Leukemias., PMID:27388473
Genetics of CD33 in Alzheimer's disease and acute myeloid leukemia., PMID:25762156
Novel drugs for older patients with acute myeloid leukemia., PMID:25142817
Targeted alpha-particle immunotherapy for acute myeloid leukemia., PMID:24857092
New agents: great expectations not realized., PMID:24309529
Precision 're'arming of CD33 antibodies., PMID:23970353
SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML., PMID:23770776
Antibody-dependent cell-mediated cytotoxicity overcomes NK cell resistance in MLL-rearranged leukemia expressing inhibitory KIR ligands but not activating ligands., PMID:23014531
Randomized phase IIb study of low-dose cytarabine and lintuzumab versus low-dose cytarabine and placebo in older adults with untreated acute myeloid leukemia., PMID:22801961
What happened to anti-CD33 therapy for acute myeloid leukemia?, PMID:22109628
Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia., PMID:20858843
5-azacytidine enhances the anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia., PMID:20495353
Gateways to clinical trials., PMID:20094643
Anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia., PMID:20065652
Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial., PMID:19557623
Newer monoclonal antibodies for hematological malignancies., PMID:18565392
Antibody therapy for acute myeloid leukemia., PMID:18381105
Integration of monoclonal antibodies and immunoconjugates into the treatment of acute myeloid leukemia., PMID:18300754
Ab therapy of AML: native anti-CD33 Ab and drug conjugates., PMID:17917880
Gateways to clinical trials., PMID:16395422
Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia., PMID:15961759
Specific targeting of CD33(+) leukemia cells by a natural killer cell line modified with a chimeric receptor., PMID:15661266
Pharmacokinetics, dosimetry, and toxicity of the targetable atomic generator, 225Ac-HuM195, in nonhuman primates., PMID:14734685
Gateways to clinical trials., PMID:12949633
Phase III trial of a humanized anti-CD33 antibody (HuM195) in patients with relapsed or refractory acute myeloid leukemia., PMID:12141950