Catalog No.
KDD16102
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD140a has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tovetumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tovetumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tovetumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
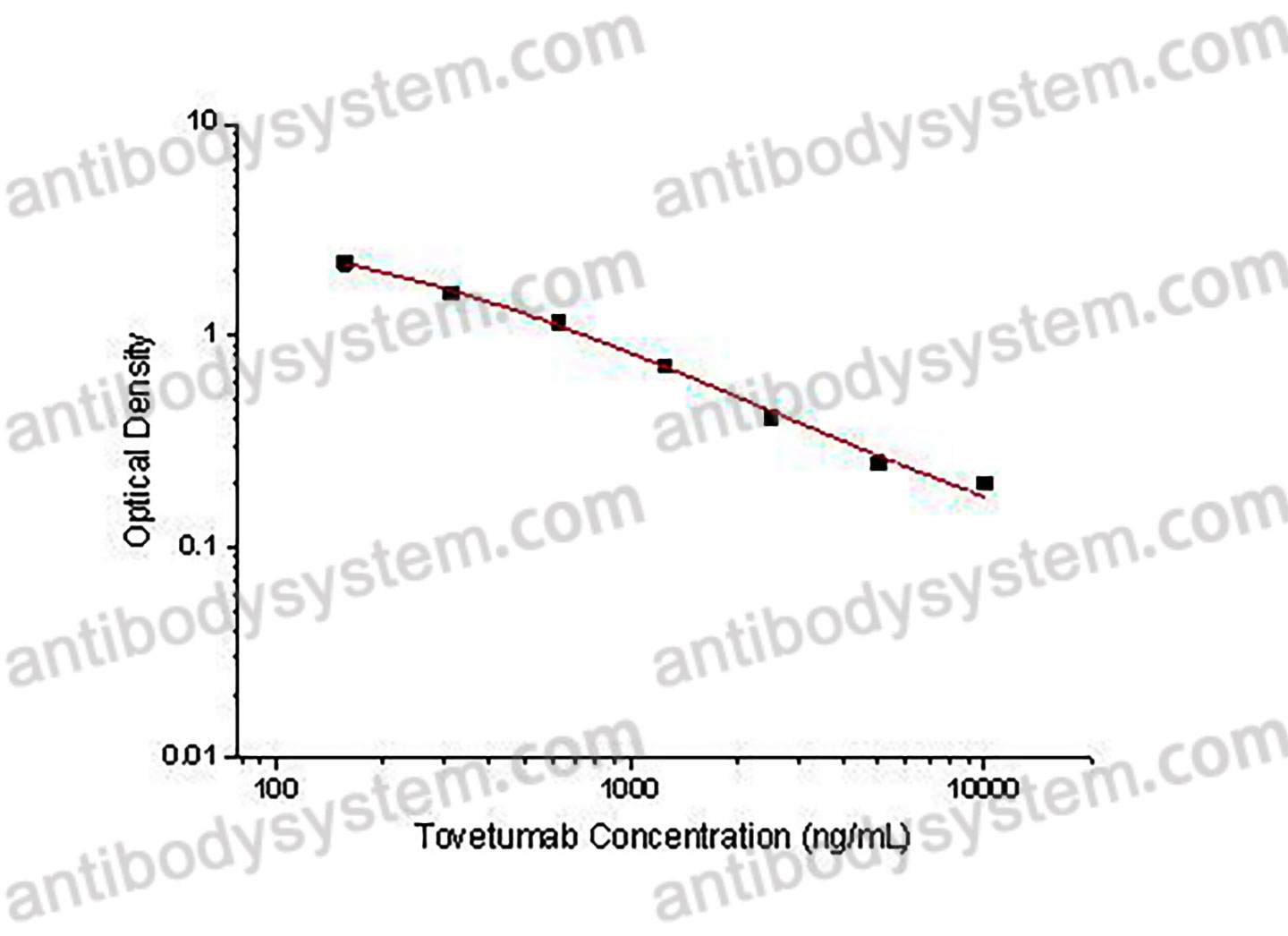
Used for the quantitative determination of Tovetumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
178.38 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
6598.2 |
1598.6 |
344.8 |
6237.4 |
1451.1 |
307.3 |
|
Standard deviation |
624.7 |
169.3 |
60.4 |
693.9 |
274.6 |
59.2 |
|
CV (%) |
9.5 |
10.6 |
17.5 |
11.1 |
18.9 |
19.3 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MEDI-575, CAS: 1243266-04-7
Background
Tovetumab (MEDI-575) is a fully human IgG2κ monoclonal antibody that specifically binds to human platelet-derived growth factor receptor alpha (PDGFRα) and blocks receptor signal transduction by PDGF ligands. The affinity of tovetumab determined using surface plasmon resonance technology and flow cytometry demonstrated comparable binding affinity for human and monkey PDGFRα. In single and repeat-dose monkey pharmacokinetic-pharmacodynamic (PK-PD) studies, tovetumab administration resulted in dose-dependent elevation of circulating levels of PDGF-AA, a member of the PDGF ligand family, due to displacement of PDGF-AA from PDGFRα by tovetumab and subsequent blockade of PDGFRα-mediated PDGF-AA degradation. As such, PDGF-AA accumulation is an indirect measurement of receptor occupancy and is a novel PD biomarker for tovetumab. The nonlinear PK of tovetumab and dose-dependent increase in circulating PDGF-AA profiles were well described by a novel mechanistic model, in which tovetumab and PDGF-AA compete for the binding to PDGFRα.