Catalog No.
KDC90701
Description
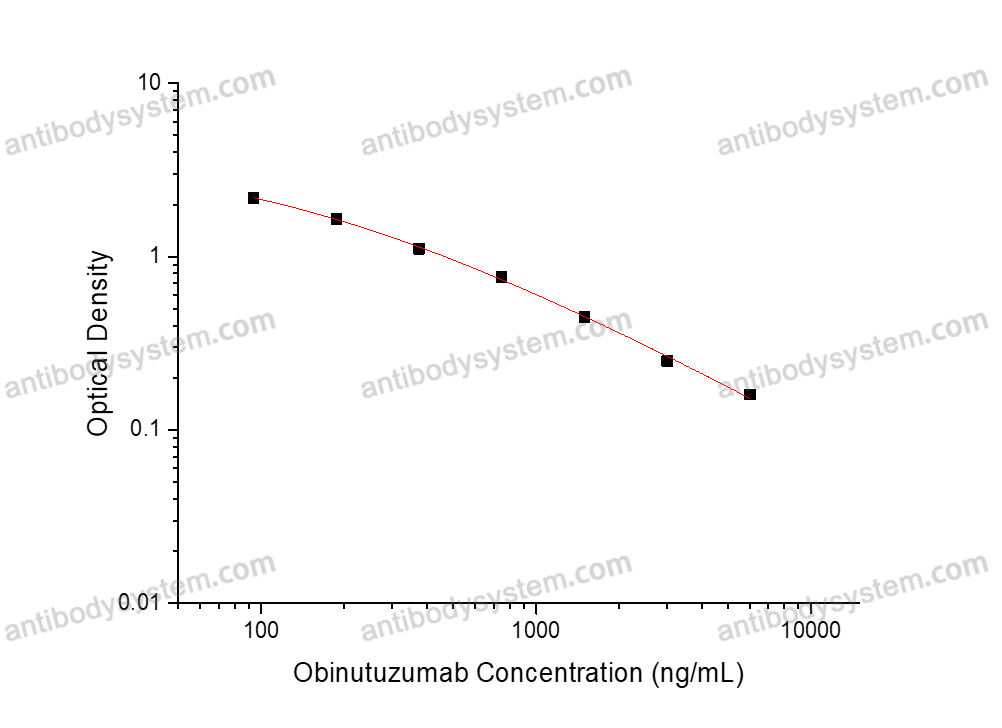
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD20 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Obinutuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Obinutuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Obinutuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Obinutuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
93.75 - 6,000 ng/mL
Sensitivity
55.62 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2986.3
|
718.1
|
162.8
|
2769.6
|
721.2
|
166.9
|
|
Standard deviation
|
245.7
|
64.8
|
27.4
|
206.5
|
48.9
|
20.2
|
|
CV (%)
|
8.2
|
9.0
|
16.8
|
7.5
|
6.8
|
12.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
GA101, RG7159, RO5072759, Afutuzumab, CAS: 949142-50-1
Beyond BCL2 (B cell lymphoma) and BTK (Bruton tyrosine kinase) inhibitors: novel agents and resistance mechanisms for chronic lymphocytic leukemia., PMID:40515863
Chemotherapy-free combination of ibrutinib and obinutuzumab for untreated advanced follicular lymphoma: results of a phase II study from the German Lymphoma Alliance., PMID:40501414
Advances and challenges in leukemia treatment: A focus on monoclonal antibodies and emerging therapies., PMID:40486885
In active lupus nephritis, adding obinutuzumab to standard therapy increased complete renal response rates at 76 wk., PMID:40456159
Short-duration infusion obinutuzumab with venetoclax in chronic lymphocytic leukemia: a prospective observational study., PMID:40453137
Single-Agent and Associated Therapies with Monoclonal Antibodies: What About Follicular Lymphoma?, PMID:40427101
Obinutuzumab in Patients With Active Lupus Nephritis: A Meta-Analysis., PMID:40424616
Obinutuzumab-Induced Inflammatory Bowel Disease-Like Colitis., PMID:40421460
Cardiac Events in Three Phase 3 Randomized Trials Including Acalabrutinib in Chronic Lymphocytic Leukemia., PMID:40413154
Successful treatment of pemphigus vulgaris with obinutuzumab., PMID:40406957
Additive Obinutuzumab Achieves High Remission Rates in Rituximab-Refractory Membranous Nephropathy., PMID:40388905
Early caplacizumab and obinutuzumab enable successful treatment of relapsing thrombotic thrombocytopenic purpura without therapeutic plasma exchange: a case report., PMID:40356910
Phase II study of venetoclax added to bendamustine and obinutuzumab in patients with high-risk follicular lymphoma as front-line therapy: PrE0403., PMID:40355425
Clinical experience and safety of venetoclax in the treatment of patients with chronic lymphocytic leukemia - real-world data from a hemato-oncology center., PMID:40353625
Obinutuzumab as a Promising Treatment for Membranous Nephropathy., PMID:40352879
CRISPRi screening identifies PIKfyve as a co-therapeutic target for obinutuzumab., PMID:40342289
Obinutuzumab is effective for the treatment of rituximab-refractory PLA2R-associated membranous nephropathy., PMID:40336511
[Seronegativity and anti-CD20: When a treatment compromises the diagnosis]., PMID:40312237
Obinutuzumab for treatment of membranous nephropathy in patients positive and negative for the phospholipase A2 receptor antibody: case reports., PMID:40303416
Obinutuzumab in Rituximab-Intolerant Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Patients., PMID:40303206
Immunotherapy for autoimmune encephalitis., PMID:40301313
Atezolizumab, obinutuzumab and venetoclax for the treatment of patients with relapsed/refractory B non-Hodgkin lymphoma: Final analysis of a phase II trial from the LYSA group., PMID:40285420
Osteonecrosis of the Jaw Associated with Obinutuzumab in a Patient with Preceding Follicular Non-Hodgkin's Lymphoma., PMID:40278317
How we manage immune-mediated thrombotic thrombocytopenic purpura after rituximab failure or intolerance., PMID:40270461
Successful Treatment of Ocular Post-Transplant Lymphoproliferative Disorder with Obinutuzumab in an 8-Year-Old Boy Following Kidney Transplant: A Case Report., PMID:40255019
An autopsy for persistent coronavirus disease 2019 pneumonia during follicular lymphoma treatment: A case report., PMID:40254437
Clinical characteristics, treatment patterns and outcomes of patients with follicular lymphoma in Colombia: a real-world evidence cohort study., PMID:40227475
Obinutuzumab and Ofatumumab are More Effective Than Rituximab in the Treatment of Membranous Nephropathy Patients With Anti-Rituximab Antibodies., PMID:40225374
Obinutuzumab-induced Acute Thrombocytopenia Mimicking Immune Thrombocytopenia in a Patient with Follicular Lymphoma., PMID:40222942
Obinutuzumab in focal segmental glomerulonephritis resistant to treatment., PMID:40221341
AMPLIFY: A second-generation BTK inhibitor for fixed-duration therapy in chronic lymphocytic leukemia., PMID:40220751
B cell depletion in lupus nephritis: From disappointments to hopes, despite some concerns., PMID:40220749
A New Pulmonary Nodule in a Patient With a History of Lymphoma., PMID:40210319
Obinutuzumab: a new frontier in the treatment of refractory idiopathic membranous nephropathy., PMID:40205126
Acalabrutinib-Obinutuzumab Improves Survival vs Chemoimmunotherapy in treatment-naive CLL in the 6-year Follow-up of ELEVATE-TN., PMID:40198878
Obinutuzumab in patients with repeated lupus nephritis flares: A case series., PMID:40186329
Glofitamab in refractory or relapsed diffuse large B cell lymphoma after failing CAR-T cell therapy: a phase 2 LYSA study., PMID:40181090
A pilot study to determine the feasibility and safety of pharmacist and nurse driven management of venetoclax ramp-up in patients with chronic lymphocytic leukemia., PMID:40170466
Treatment of nephrotic syndrome with anti-CD20 therapies in pregnancy: a case series and review of the literature., PMID:40148078
Preclinical B cell depletion and safety profile of a brain-shuttled crystallizable fragment-silenced CD20 antibody., PMID:40118783
Zanubrutinib, venetoclax, and obinutuzumab in R/R CLL., PMID:40111334
The role of obinutuzumab in rituximab-refractory membranous nephropathy and minimal change disease., PMID:40104551
Long-term outcomes of chemoimmunotherapy with obinutuzumab/chlorambucil in chronic lymphocytic leukemia., PMID:40101233
Erratum: Phase II Study of Acalabrutinib, Venetoclax, and Obinutuzumab in a Treatment-Naïve Chronic Lymphocytic Leukemia Population Enriched for High-Risk Disease., PMID:40101168
Upfront fixed-duration treatment strategies for chronic lymphocytic leukemia in Arab populations: a position statement from the Gulf region., PMID:40078401
Real-World Effectiveness of Frontline Treatments Among Patients with Chronic Lymphocytic Leukemia: Results from ConcertAI., PMID:40075647
Anti-CD20 maintenance strategies to face the challenge of COVID-19 pandemic in follicular lymphoma: results from the R-FolSTOP multicentre Italian study., PMID:40074837
Bilateral drusenoid deposits with subretinal fluid and cystoid macular edema in a serum anti-phospholipase A2 receptor antibody positive patient: A case report., PMID:40061915
[The combined regimen based on obinutuzumab plus glucocorticoid for 4 cases of relapsed iTTP]., PMID:40059685
Obinutuzumab effective for lupus nephritis., PMID:40055568