Catalog No.
KDC82403
Description
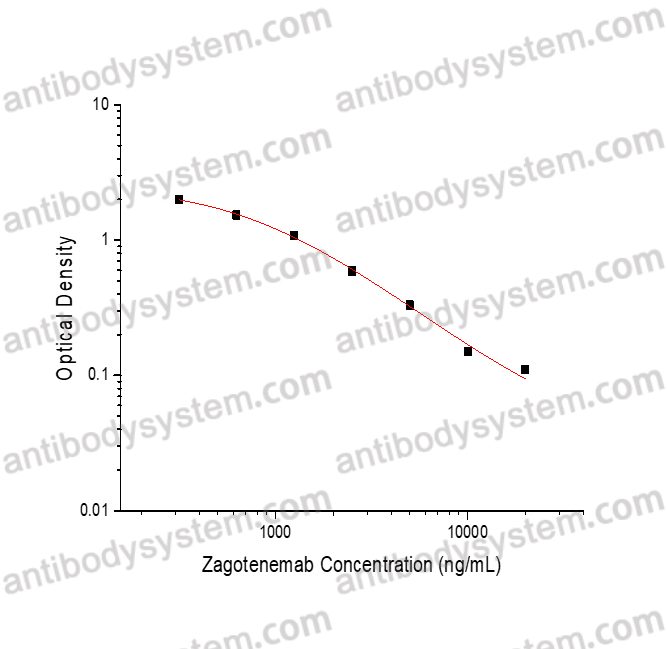
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human MAPT has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Zagotenemab in the sample competitively binds to the pre-coated protein with biotin-labeled Zagotenemab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Zagotenemab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Zagotenemab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
312.5 - 20,000 ng/mL
Sensitivity
61.51 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
11004.1 |
2714.5 |
728.1 |
11463.8 |
2738.7 |
668.9 |
|
Standard deviation |
571.4 |
139.7 |
61.3 |
1803.1 |
133.6 |
97.0 |
|
CV (%) |
5.2 |
5.1 |
8.4 |
15.7 |
4.9 |
14.5 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
LY-3303560, CAS: 2019133-28-7
Background
Zagotenemab is a humanized anti-tau antibody derived from MCI-1, Peter Davies' mouse monoclonal antibody against an early pathological conformation of tau. Zagotenemab binds and neutralizes soluble tau aggregates.Preclinical studies in tau transgenic mice showed that immunotherapy with MCI-1 reduced levels of hyperphosphorylated insoluble tau levels and neurofibrillary pathology (Chai et al., 2011).At the 2017 AAIC meeting, Lilly presented data from surface plasmon resonance binding and ELISA assays to assess LY3303560's selectivity to aggregates over monomer and characterize its epitope. LY3303560 reportedly had high affinity to soluble tau aggregate in vitro, at a KD of below 220 picomolar, compared to monomer, KD of 235 nanomolar. The antibody reportedly recognizes a conformational epitope whose primary epitope is in tau's N-terminal region. Intravenous administration to monkeys indicated clearance of 0.15 ml/h/kg and a half-life of 13 days. SC administration indicated a bioavailability of 79 percent, and rat CSF concentration was 0.1 percent of plasma at 24 hours after IV administration (Alam et al., 2017).