Catalog No.
KDC42101
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant Human CSF1 has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Lacnotuzumab present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Lacnotuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
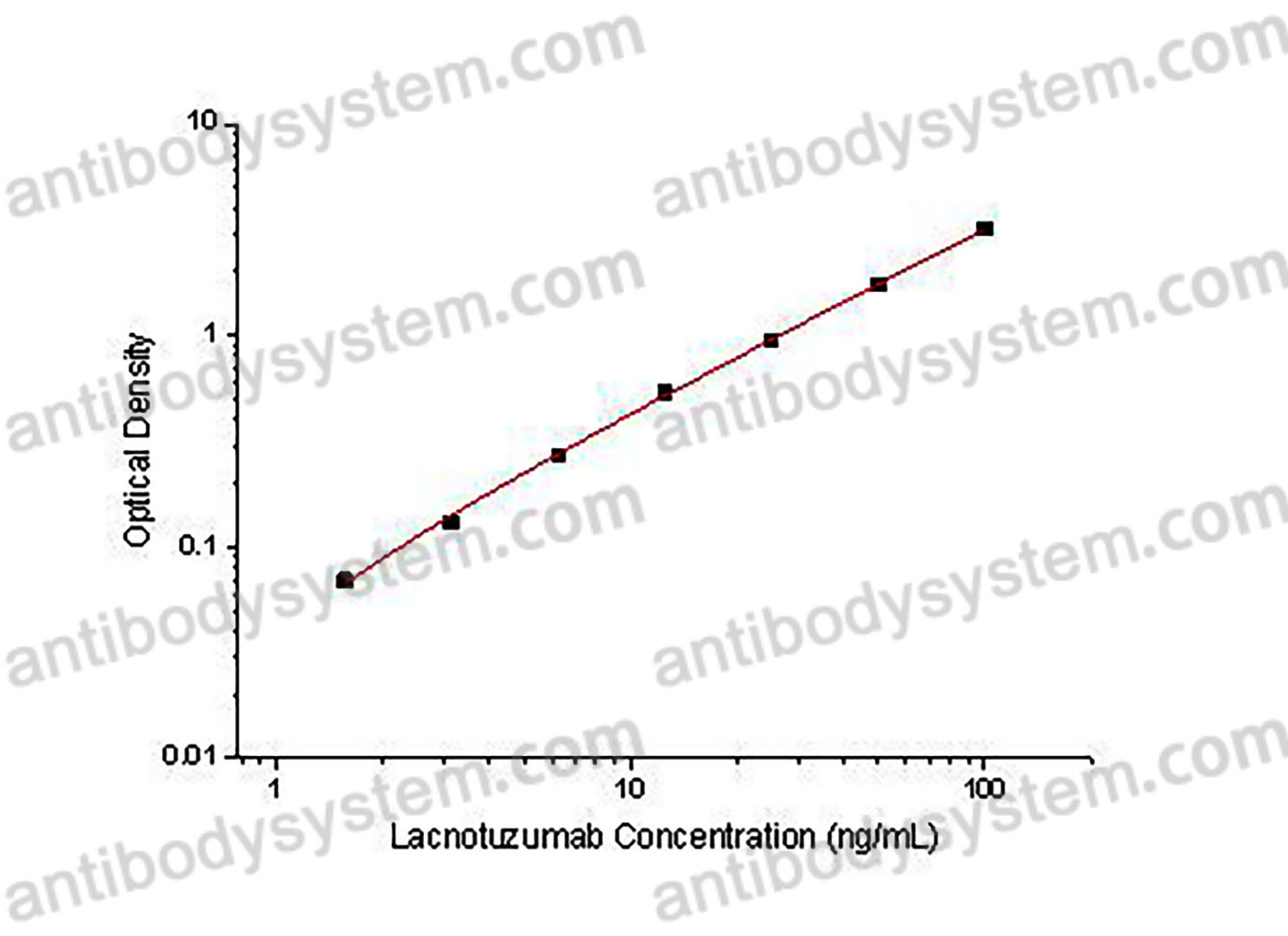
Used for the quantitative determination of Lacnotuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.95 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
42.0 |
11.0 |
2.6 |
43.9 |
11.6 |
2.9 |
|
Standard deviation |
2.2 |
0.4 |
0.1 |
2.3 |
0.7 |
0.3 |
|
CV (%) |
5.3 |
4.1 |
5.3 |
5.3 |
5.8 |
10.1 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
MCS-110, CAS: 1831128-32-5
Background
Lacnotuzumab, as known as MCS110, is a novel, high-affinity, humanized, anti-CSF-1 monoclonal antibody that prevents CSF-1 from activating the CSF-1R. The drug is being developed by Novartis Europharm Limited. On 15 October 2014, orphan designation (EU/3/14/1350) was granted by the European Commission to Novartis for recombinant human monoclonal antibody of the IgG1 kappa class against human macrophage colony-stimulating factor for the treatment of tenosynovial giant cell tumor, localized and diffuse type. They conducted a phase 1 study to assess the safety and tolerability of administering single ascending doses and repeat doses of lacnotuzumab to healthy volunteers, and to examine the pharmacokinetics (PK), pharmacodynamics (PD), and mechanistic properties of lacnotuzumab. Nonclinical investigations of the mechanism of action of lacnotuzumab were also performed to explain the observed adverse events (AEs) profile associated with CSF-1 pathway inhibition. Lacnotuzumab was generally well tolerated. The majority of AEs were low grade, no unexpected or novel AEs were observed, and there were no discontinuations for AEs. Lacnotuzumab also showed dose-dependent, on-target effects on multiple downstream biomarkers. Preclinical investigations of the CK elevation and periorbital swelling observed after lacnotuzumab administration suggest that these are reversible, nonpathological events linked to inhibition of the CSF-1 pathway. These data support further evaluation of lacnotuzumab in clinical studies.