Catalog No.
KDC25202
Description
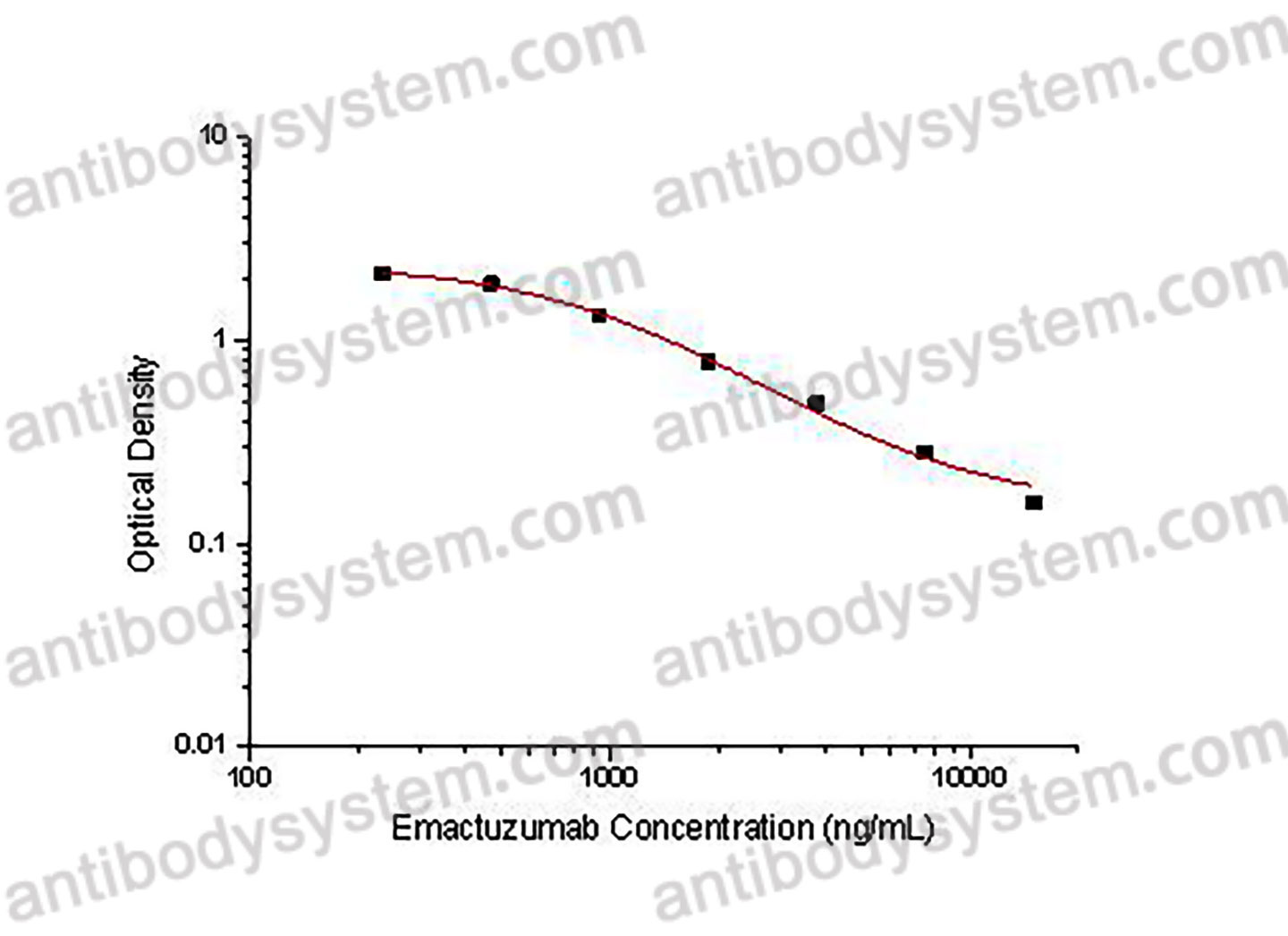
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD115 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Emactuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Emactuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Emactuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Emactuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
234.38 - 15,000 ng/mL
Sensitivity
35.75 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
7197.4 |
1658.1 |
354.1 |
7757.5 |
1737.2 |
441.6 |
|
Standard deviation |
308.8 |
152.2 |
33.5 |
786.5 |
221.6 |
76.8 |
|
CV (%) |
4.3 |
9.2 |
9.5 |
10.1 |
12.8 |
17.4 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
RG 7155, RO5509554, CAS: 1448221-67-7
Background
Emactuzumab, also known as RG-7155, is a humanized monoclonal antibody developed by Genentech/Roche. It was designed to directly against the tyrosine kinase receptor colony stimulating factor 1 receptor (CSF1R), which is also known as macrophage colony-stimulating factor receptor (M-CSFR) and Cluster of Differentiation 115 (CD115). Emactuzumab has been investigated in the treatment for the diseases including fallopian tube cancer, ovarian cancer, peritoneal cancer and solid tumors. The trail that emactuzumab combination regimen with selicrelumab (RG7876) in solid tumors is currently in Phase II.