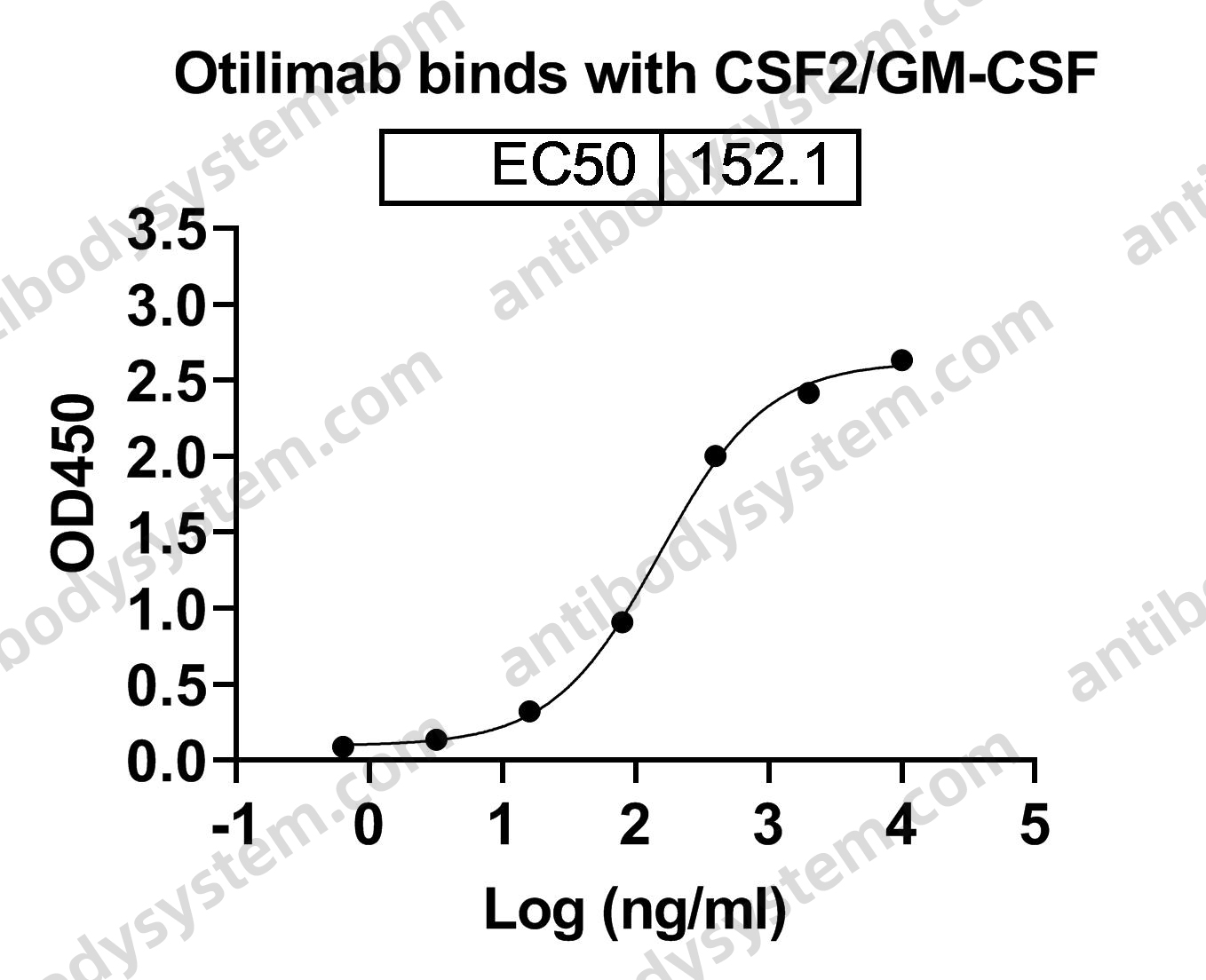
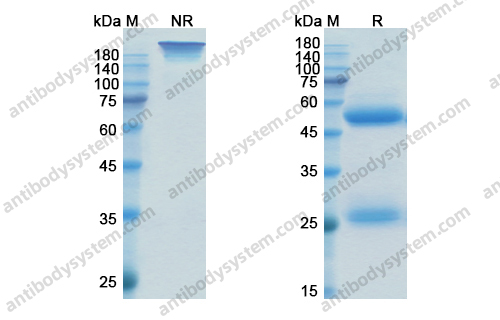
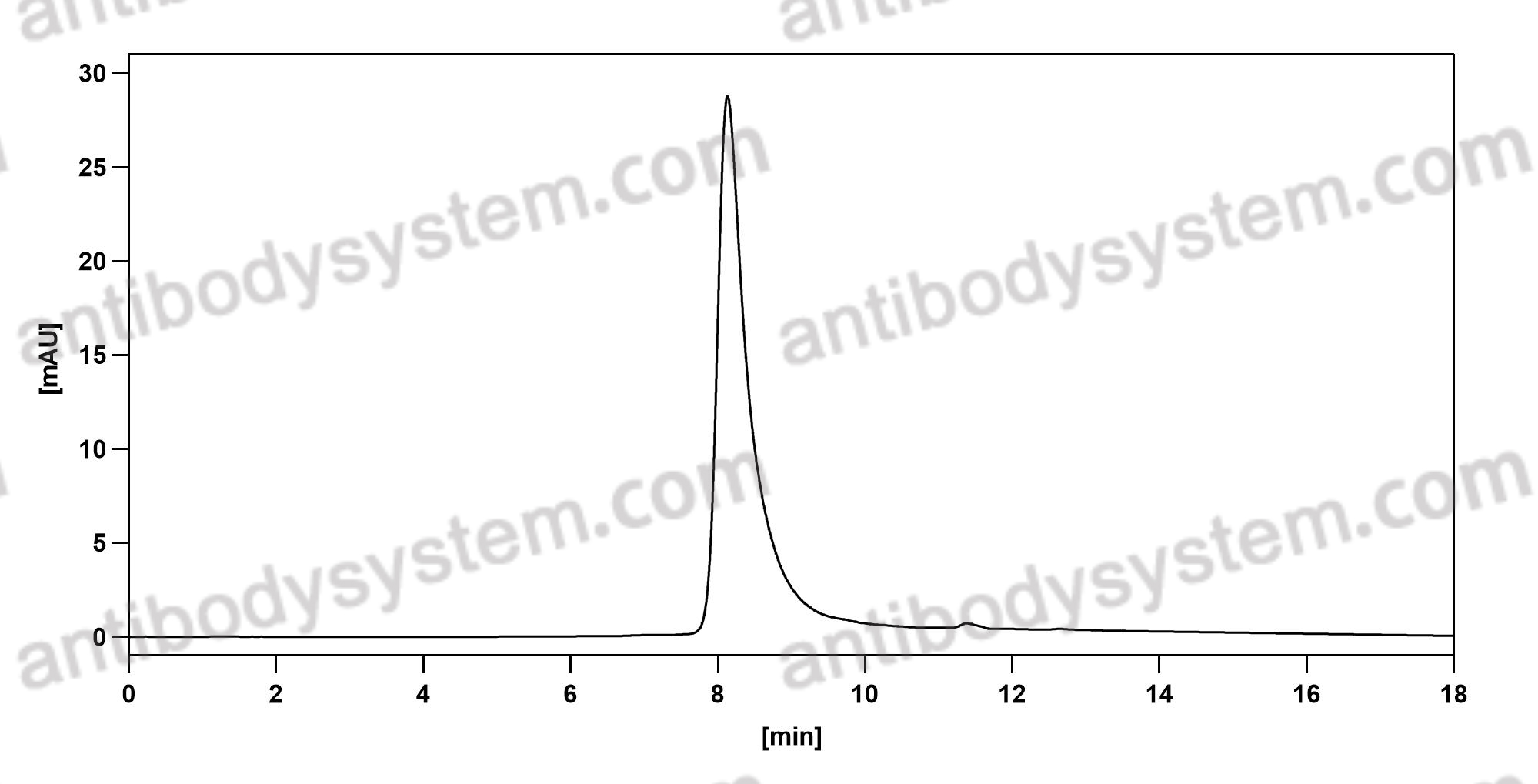
Catalog No.
DHC06702
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
Granulocyte-macrophage colony-stimulating factor, Sargramostim, GMCSF, CSF, Colony-stimulating factor, CSF2, GM-CSF, Molgramostin
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
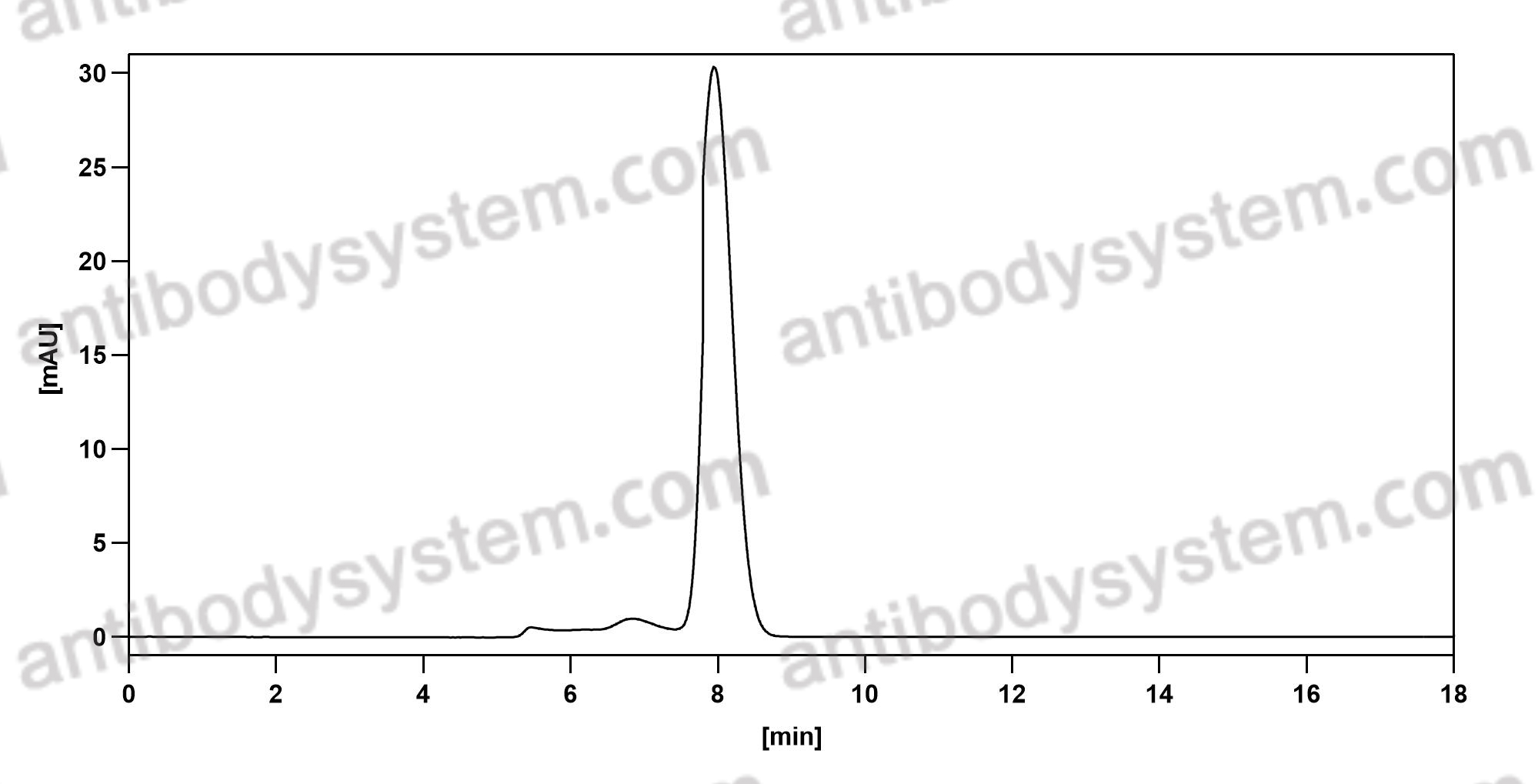
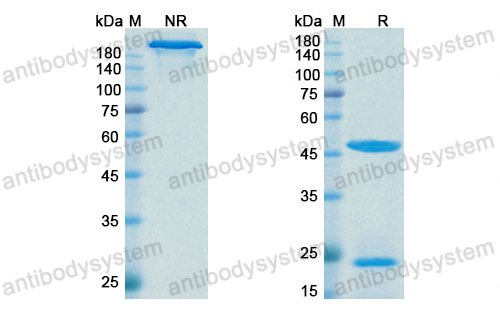
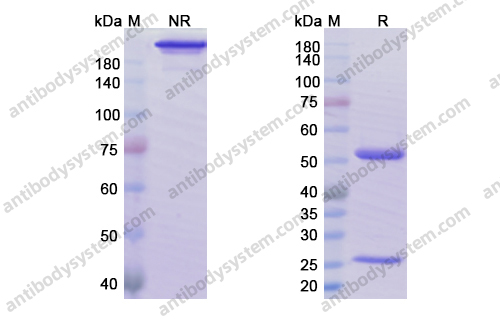
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P04141
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MOR-04357, 3196165, GSK3196165, MOR103, CAS: 1638332-55-4
Clone ID
Otilimab
Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis, PMID: 32033937
MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial, PMID: 24534756
Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects, PMID: 31486005
Long-term safety and efficacy of anti-GM-CSF otilimab in patients with rheumatoid arthritis: long-term extension of three phase 3 randomised trials (contRAst X)., PMID:40044198
Meaningful score changes for SF-36v2, FACIT-fatigue, and RASIQ in rheumatoid arthritis., PMID:38252223
Anti-GM-CSF otilimab versus tofacitinib or placebo in patients with active rheumatoid arthritis and an inadequate response to conventional or biologic DMARDs: two phase 3 randomised trials (contRAst 1 and contRAst 2)., PMID:37699654
Anti-GM-CSF otilimab versus sarilumab or placebo in patients with rheumatoid arthritis and inadequate response to targeted therapies: a phase III randomised trial (contRAst 3)., PMID:37696589
Severe SARS-CoV-2 Pneumonia and Pneumomediastinum/Pneumothorax: A Prospective Observational Study in an Intermediate Respiratory Care Unit., PMID:37306158
GM-CSF targeting in COVID-19: an approach based on fragile foundations., PMID:36396141
A randomised trial of anti-GM-CSF otilimab in severe COVID-19 pneumonia (OSCAR)., PMID:36229048
A guide to immunotherapy for COVID-19., PMID:35064248
Efficacy, patient-reported outcomes, and safety of the anti-granulocyte macrophage colony-stimulating factor antibody otilimab (GSK3196165) in patients with rheumatoid arthritis: a randomised, phase 2b, dose-ranging study., PMID:38279364
MRI of the joint and evaluation of the granulocyte-macrophage colony-stimulating factor-CCL17 axis in patients with rheumatoid arthritis receiving otilimab: a phase 2a randomised mechanistic study., PMID:38279363
Anti-granulocyte-macrophage colony-stimulating factor antibody otilimab in patients with hand osteoarthritis: a phase 2a randomised trial., PMID:38273625
Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis., PMID:32033937
Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects., PMID:31486005
MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial., PMID:24534756