Catalog No.
KDC15802
Description
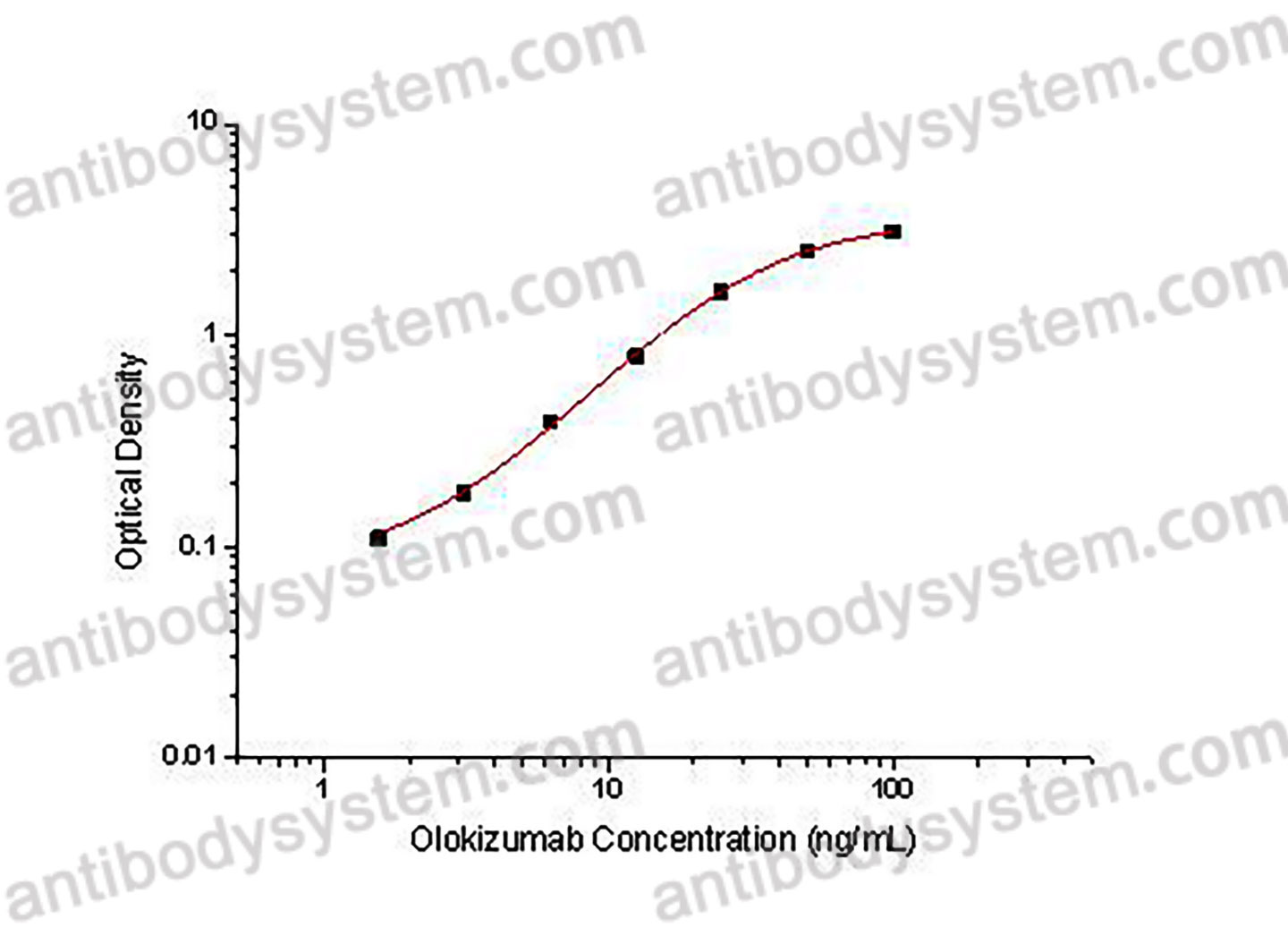
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant Human IL6 has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Olokizumab present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Olokizumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Olokizumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.74 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
49.2
|
12.4
|
5.8
|
44.6
|
11.5
|
5.9
|
|
Standard deviation
|
3.7
|
0.4
|
0.3
|
2.6
|
0.4
|
0.4
|
|
CV (%)
|
7.5
|
2.9
|
5.0
|
5.8
|
3.7
|
6.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CDP 6038, CAS: 1007223-17-7
Background
Olokizumab (CDP6038), a humanized monoclonal antibody (mAb) specific for the interleukin-6 (IL-6) cytokine, is currently in development for the treatment of rheumatoid arthritis (RA). It targets the IL-6 cytokine rather than the receptor, and selectively blocks the final assembly of the signalling complex. In Phase I (healthy volunteers) and IIa (patients with RA on MTX) clinical trials, olokizumab was well tolerated after intravenous and subcutaneous delivery with a median plasma half-life of approximately 31 days, 76% bioavailability and no apparent antidrug antibody-mediated clearance. Olokizumab also markedly reduced free IL-6 levels and suppressed C-reactive protein (CRP) up to 12 weeks after single-dose subcutaneous administration in patients with RA.
Correction: Olokizumab plus methotrexate: safety and efficacy over 106 weeks of treatment., PMID:39919910
EFFICACY AND SAFETY OF OLOKIZUMAB IN THE MANAGEMENT OF RHEUMATOID ARTHRITIS- A SYSTEMATIC REVIEW AND META-ANALYSIS., PMID:39585284
Olokizumab effect on chronic pain in rheumatoid arthritis: Results of the PROLOGUE observational study., PMID:39441547
Efficacy and safety of current therapies for difficult-to-treat rheumatoid arthritis: a systematic review and network meta-analysis., PMID:39198829
Identification of critical genes and metabolic pathways in rheumatoid arthritis and osteoporosis toward drug repurposing., PMID:39079412
Inflammation-mediated drug interactions of olokizumab and cytochrome P450 activities in patients with rheumatoid arthritis., PMID:38984761
Results of a 12-Week Open-Label, Non-Interventional Study of the Efficacy and Safety of Olokizumab Therapy in Patients with Rheumatoid Arthritis after Switching from Anti-B-Cell Therapy during the SARS-CoV-2 Pandemic., PMID:38955912
Olokizumab plus methotrexate: safety and efficacy over 106 weeks of treatment., PMID:38955475
Efficacy of Olokizumab against Comorbid Depressive Disorder in Patients with Rheumatoid Arthritis: Preliminary Results of the Study., PMID:38861142
Text Mining and Drug Discovery Analysis: A Comprehensive Approach to Investigate Diabetes-Induced Osteoporosis., PMID:38250601
Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What Have We Learned?, PMID:37989892
Comparison of the efficacy and safety of olokizumab at different dosages in patients with active rheumatoid arthritis: a network meta-analysis of randomized controlled trials., PMID:37266677
Interleukin-6 blocking agents for treating COVID-19: a living systematic review., PMID:37260086
[Clinical cases of complicated diverticulitis against the background of severe course COVID-19. Case report]., PMID:37167171
NEUROPROTECTIVE AND ANTIOXIDANT POTENTIAL OF MONTELUKAST-ACETYLCYSTEINE COMBINATION THERAPY FOR BRAIN PROTECTION IN PATIENTS WITH COVID-19 INDUCED PNEUMONIA., PMID:37042600
Targeting Olokizumab-Interleukin 6 interaction interface to discover novel IL-6 inhibitors., PMID:36995131
Identification of potential molecular mechanisms and candidate drugs for radiotherapy- and chemotherapy-induced mucositis., PMID:36939936
The efficacy and safety of olokizumab for rheumatoid arthritis: a systematic review, pairwise, and network meta-analysis., PMID:36792848
Comparison of the efficacy and safety of tocilizumab, sarilumab, and olokizumab in patients with active rheumatoid arthritis: a network meta-analysis of randomized controlled trials., PMID:36607422
Olokizumab's Effectiveness and Safety in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials., PMID:36535857
Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis., PMID:36368906
Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study., PMID:36109142
Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis., PMID:36001712
Drug discovery in spinal cord injury-induced osteoporosis: a text mining-based study., PMID:35957736
Genetic and Clinical Factors Associated with Olokizumab Treatment in Russian Patients with Rheumatoid Arthritis., PMID:35455757
A Novel Humanized Anti-Interleukin-6 Antibody HZ0408b With Anti-Rheumatoid Arthritis Therapeutic Potential., PMID:35126375
Impact of olokizumab on platelets, leukocytes and erythrocytes during mild COVID-19., PMID:34565057
Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study., PMID:34344706
What do we know about IL-6 in COVID-19 so far?, PMID:37287491
Long-term safety and efficacy of olokizumab in patients with rheumatoid arthritis and inadequate response to tumor necrosis factor inhibitor therapy in phase II studies., PMID:34101570
Interleukin-6 blocking agents for treating COVID-19: a living systematic review., PMID:33734435
[Experience of olokizumab use in COVID-19 patients]., PMID:33720587
Antibodies to watch in 2021., PMID:33459118
Analysis of miRNA Expression in Patients with Rheumatoid Arthritis during Olokizumab Treatment., PMID:33142700
Biosensing Cytokine IL-6: A Comparative Analysis of Natural and Synthetic Receptors., PMID:32847008
Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis., PMID:32033937
Interleukin-6 inhibition in the management of non-infectious uveitis and beyond., PMID:31523783
Rheumatoid arthritis: new monoclonal antibodies., PMID:29771256
[Anti-IL-6 : new therapeutic trends]., PMID:28703549
Profile of sarilumab and its potential in the treatment of rheumatoid arthritis., PMID:28579757
[IL-6 blockade]., PMID:27311186
Probing binding mechanism of interleukin-6 and olokizumab: in silico design of potential lead antibodies for autoimmune and inflammatory diseases., PMID:26982101
Targeting interleukin-6 for noninfectious uveitis., PMID:26392750
Efficacy and safety of olokizumab in Asian patients with moderate-to-severe rheumatoid arthritis, previously exposed to anti-TNF therapy: Results from a randomized phase II trial., PMID:26358841
IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future., PMID:25648633
Safety and pharmacokinetics of olokizumab, an anti-IL-6 monoclonal antibody, administered to healthy male volunteers: A randomized phase I study., PMID:27129012
Model-Based Optimal Design and Execution of the First-Inpatient Trial of the Anti-IL-6, Olokizumab., PMID:24941311
IL-6 targeting compared to TNF targeting in rheumatoid arthritis: studies of olokizumab, sarilumab and sirukumab., PMID:24833786
Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling., PMID:24670876
Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study., PMID:24641941