Catalog No.
KDC09601
Description
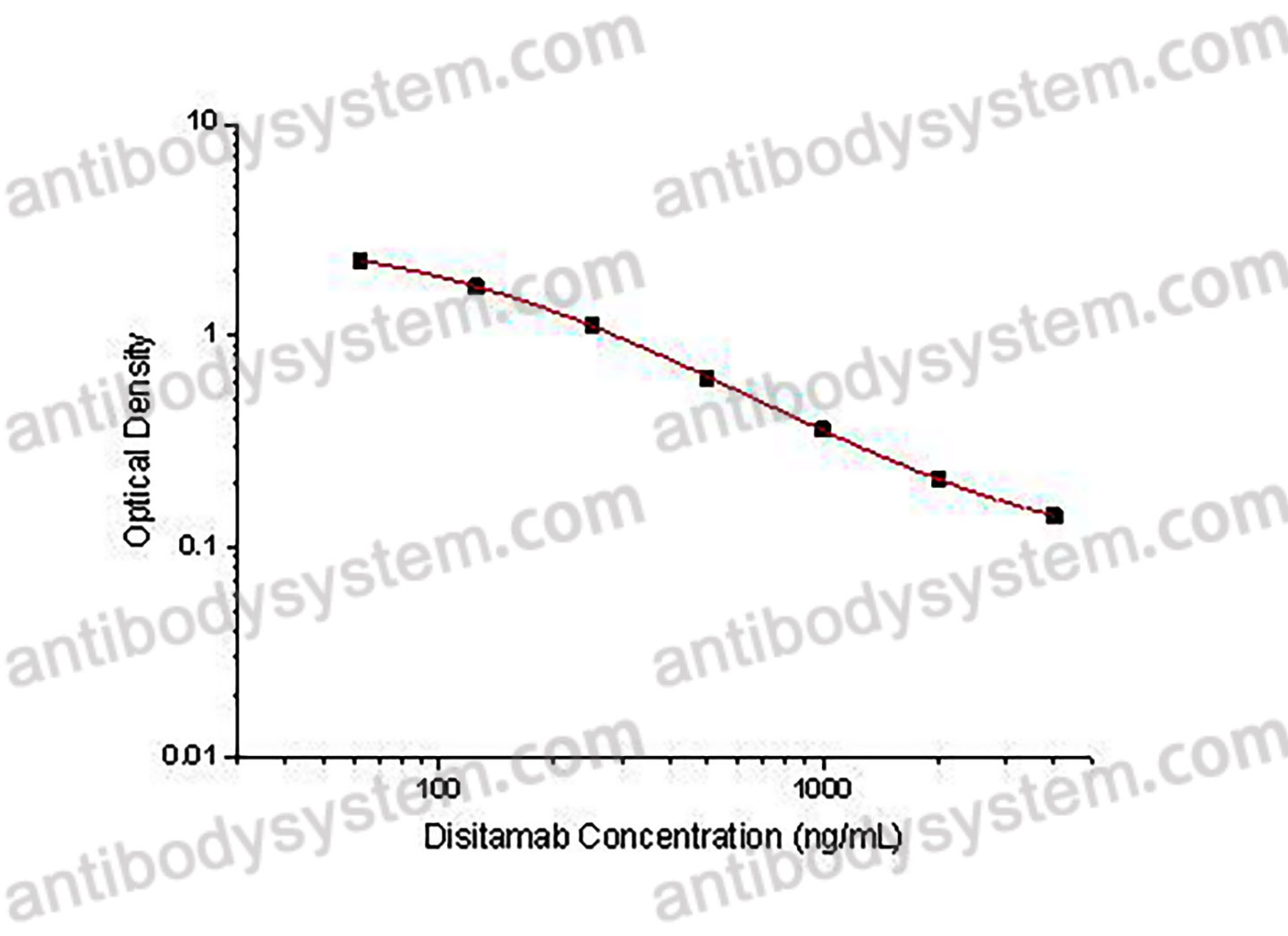
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD340 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Disitamab in the sample competitively binds to the pre-coated protein with biotin-labeled Disitamab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Disitamab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Disitamab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
62.5 - 4,000 ng/mL
Sensitivity
43.24 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2179.9
|
594.6
|
142.6
|
2360.7
|
584.5
|
146.7
|
|
Standard deviation
|
272.7
|
112.7
|
14.2
|
388.2
|
47.2
|
22.1
|
|
CV (%)
|
12.5
|
19.0
|
10.0
|
16.4
|
8.1
|
15.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
RC48-0, Disitamab Vedotin, CAS: 2185868-98-6
Background
Disitamab vedotin (Aidixi®) is an antibody-drug conjugate comprising a monoclonal antibody against human epidermal growth factor receptor 2 (HER2) conjugated via a cleavable linker to the cytotoxic agent monomethyl auristatin E. Disitamab vedotin is being developed by RemeGen for the treatment of solid tumours, including gastric cancer; Seagen has the right to develop disitamab vedotin globally outside of RemeGen's territory. In June 2021, disitamab vedotin received its first Biologics License Application (BLA) approval in China for the treatment of patients with HER2-overexpressing (defined as IHC2+ or 3+) locally advanced or metastatic gastric cancer (including gastroesophageal junction adenocarcinoma) who have received at least two systemic chemotherapy regimens. Disitamab vedotin as monotherapy or combination therapy is also in clinical development for the treatment of other solid tumours globally, including urothelial cancer in China and the USA, and biliary tract cancer, non-small cell lung cancer and HER2-positive and HER2-low expressing breast cancer in China. This article summarizes the milestones in the development of disitamab vedotin leading to this first approval for locally advanced or metastatic gastric cancer.
The role of HER2-targeted therapy in urothelial carcinomas., PMID:40478157
Efficacy and biomarker analysis of neoadjuvant disitamab vedotin (RC48-ADC) combined immunotherapy in patients with muscle-invasive bladder cancer: A multi-center real-world study., PMID:40469503
EXPRESS: Advanced Therapies for Stomach Cancer., PMID:40448345
Antibody-drug conjugate (disitamab vedotin) therapy targeting HER2-low or higher advanced extramammary Paget's disease., PMID:40421956
Progress of antibody-drug conjugates in the treatment of locally advanced or metastatic urothelial carcinoma: opportunities and challenges., PMID:40377724
Evaluating the efficacy and safety of bladder-sparing regimen with Disitamab Vedotin combined with Toripalimab and pelvic lymph node dissection in muscle-invasive bladder cancer patients: study protocol of a multicenter single-arm phase II trial., PMID:40361038
Preclinical pharmacokinetics, distribution, metabolism and excretion of disitamab vedotin., PMID:40314006
RC48-ADC monotherapy or in combination with immunotherapy for locally advanced or metastatic urothelial carcinoma with HER2 low and null expression: a multicenter, real-world, retrospective study., PMID:40307755
Efficacy of disitamab vedotin-containing therapy in metastatic colorectal cancer: A case report., PMID:40130050
Efficacy and safety of DV in HER2-negative and HER2-low locally advanced or metastatic urothelial carcinoma: Results of a phase 2 study., PMID:40112819
Disitamab vedotin vs. gemcitabine-cisplatin regimen with immunotherapy: a comparative analysis of efficacy and safety in muscle-invasive bladder cancer., PMID:40083552
Efficacy of disitamab vedotin (RC48) for previously treated human epidermal growth factor receptor 2-positive breast cancer with symptomatic brain metastases: a case report and review of the literature., PMID:40063535
Efficacy and safety of disitamab vedotin in combination with immune checkpoint inhibitors in patients with locally advanced or metastatic urothelial carcinoma., PMID:40057923
Disitamab vedotin combined with pyrotinib as salvage treatment in Her-2-amplified treatment refractory metastatic colorectal cancer: a case report., PMID:40051563
Severe cutaneous drug toxicity following disitamab vedotin treatment for metastatic gastric cancer: a case report., PMID:39886664
The combination treatment of RC48 and STAT3 inhibitor acts as a promising therapeutic strategy for basal bladder cancer., PMID:39840049
Disitamab vedotin in preclinical models of HER2-positive breast and gastric cancers resistant to trastuzumab emtansine and trastuzumab deruxtecan., PMID:39837059
A retrospective multicenter study on the efficacy and safety of disitamab vedotin monotherapy versus combination with anti-PD-1 immunotherapy in advanced gastric cancer., PMID:39825061
Application of disitamab vedotin in the multiline treatment of EGFR mutation-positive lung adenocarcinoma with Her-2 overexpression., PMID:39726714
Disitamab vedotin plus toripalimab in patients with locally advanced or metastatic urothelial carcinoma (RC48-C014): a phase Ib/II dose-escalation and dose-expansion study., PMID:39662628
Efficacy and safety of RC48-ADC in HER2-positive and HER2-low metastatic breast cancer: a multicenter, real-world study., PMID:39582543
Efficacy of disitamab vedotin in non-small cell lung cancer with HER2 alterations: a multicenter, retrospective real-world study., PMID:39568568
Real-world application of disitamab vedotin (RC48-ADC) in patients with breast cancer with different HER2 expression levels: efficacy and safety analysis., PMID:39550213
Antibody-Drug Conjugates in Breast Cancer: Toward a Molecular Perspective Into Clinical Practice., PMID:39475662
Disitamab vedotin combined with apatinib in gastric cancer: A case report and review of literature., PMID:39473863
FOXA1, induced by RC48, regulates HER2 transcription to enhance the tumorigenic capacity of lung cancer through PI3K/AKT pathway., PMID:39440051
Case report: Successful treatment of advanced urothelial carcinoma with trophoblastic differentiation using Tislelizumab and Disitamab vedotin., PMID:39397900
Complete response to disitamab vedotin in HER2-low metastatic endometrial carcinoma: a case report and review of the literature., PMID:39351350
Disitamab vedotin (RC48) long-term regimen in a post-nephroureterectomy patient with metastases: a case report., PMID:39346730
In-depth characterization of a cysteine-linked ADC disitamab vedotin by multiple LC-MS analysis methods and cutting-edge imaged capillary isoelectric focusing coupled with native mass spectrometry., PMID:39270568
Case report: Disitamab vedotin combined with immunotherapy demonstrated excellent efficacy in scrotal Paget's disease with Her-2 overexpression., PMID:38989283
Partial response to trastuzumab deruxtecan (DS8201) following progression in HER2-amplified breast cancer with pulmonary metastases managed with disitamab vedotin (RC48): a comprehensive case report and literature review., PMID:38952555
Disitamab vedotin, a HER2-directed antibody-drug conjugate, in patients with HER2-overexpression and HER2-low advanced breast cancer: a phase I/Ib study., PMID:38940019
Systematic review of recent advancements in antibody-drug and bicycle toxin conjugates for the treatment of urothelial cancer., PMID:38779496
Assessment of HER2 status in extramammary Paget disease and its implication for disitamab vedotin, a novel humanized anti-HER2 antibody-drug conjugate therapy., PMID:38743132
Elderly patients with advanced HER2-positive breast cancer with liver metastases benefit from low dose disitamab vedotin (RC48): case series and literature review., PMID:38718261
Disitamab Vedotin Alone or in Combination With Immune Checkpoint Inhibitors in Bladder-Sparing Treatment of Muscle-Invasive Bladder Cancer: A Real-World Study., PMID:38636170
Optimal Sequential Strategies for Antibody-Drug Conjugate in Metastatic Breast Cancer: Evaluating Efficacy and Cross-Resistance., PMID:38574190
Implementation of antibody-drug conjugates in HER2-positive solid cancers: Recent advances and future directions., PMID:38565055
Development and validation of bioanalytical methods to support clinical study of disitamab vedotin., PMID:38530234
Downstaging guided neoadjuvant strategy shift and bladder preservation in locally advanced bladder cancer: A case report., PMID:38515680
Expression of HER2 in high-grade urothelial carcinoma based on Chinese expert consensus and the clinical effects of disitamab vedotin-tislelizumab combination therapy in the treatment of advanced patients., PMID:38455962
Efficacy and safety of disitamab vedotin after trastuzumab for HER2 positive breast cancer: a real-world data of retrospective study., PMID:38455400
Comparison of trastuzumab emtansine, trastuzumab deruxtecan, and disitamab vedotin in a multiresistant HER2-positive breast cancer lung metastasis model., PMID:38367127
mTOR inhibitor introduce disitamab vedotin (RC48-ADC) rechallenge microtubule-chemotherapy resistance in HER2-low MBC patients with PI3K mutation., PMID:38344201
RC48-ADC combined with tislelizumab as neoadjuvant treatment in patients with HER2-positive locally advanced muscle-invasive urothelial bladder cancer: a multi-center phase Ib/II study (HOPE-03)., PMID:38269021
Long-term survival in a patient with multiple metastatic gastric cancer treated with PTX plus emvolimab and disitamab vedotin: case report and treatment experience: A case report., PMID:38241572
Disitamab vedotin (RC48) plus toripalimab for HER2-expressing advanced gastric or gastroesophageal junction and other solid tumours: a multicentre, open label, dose escalation and expansion phase 1 trial., PMID:38235421
Efficacy and safety analysis of a HER2-targeting antibody-drug conjugate combined with immune checkpoint inhibitors in solid tumors: a real-world study., PMID:38147019
Immune checkpoint inhibitors enhanced the antitumor efficacy of disitamab vedotin for patients with HER2-positive or HER2-low advanced or metastatic gastric cancer: a multicenter real-world study., PMID:38102538