Catalog No.
KDC06701
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CSF2 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Lenzilumab in the sample competitively binds to the pre-coated protein with biotin-labeled Lenzilumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Lenzilumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
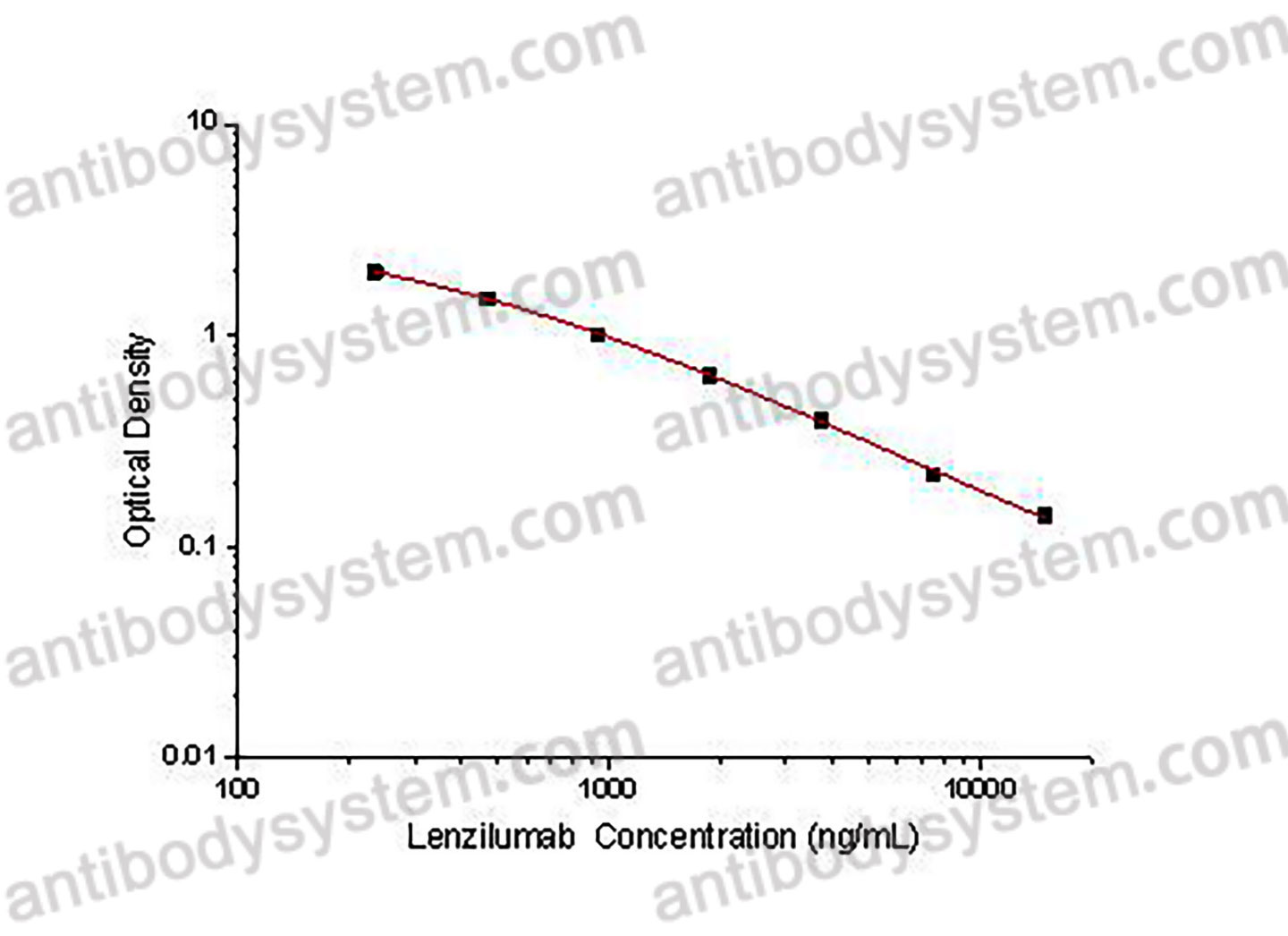
Used for the quantitative determination of Lenzilumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
234.38 - 15,000 ng/mL
Sensitivity
118.12 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
7024.1 |
1780.5 |
485.3 |
7028.7 |
2140.8 |
563.4 |
|
Standard deviation |
446.5 |
72.5 |
65.1 |
430.6 |
119.2 |
78.2 |
|
CV (%) |
6.4 |
4.1 |
13.4 |
6.1 |
5.6 |
13.9 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
KB-003, CAS: 1229575-09-0
Background
Lenzilumab is a novel Humaneered ® anti-human GM-CSF monoclonal antibody that directly binds GM-CSF and prevents signaling through its receptor. The LIVE-AIR Phase 3 randomized, double-blind, placebo-controlled trial investigated the efficacy and safety of lenzilumab to assess the potential for lenzilumab to improve the likelihood of ventilator-free survival (referred to herein as survival without ventilation, SWOV), beyond standard supportive care, in hospitalized subjects with severe COVID-19.