Catalog No.
KDB95802
Description
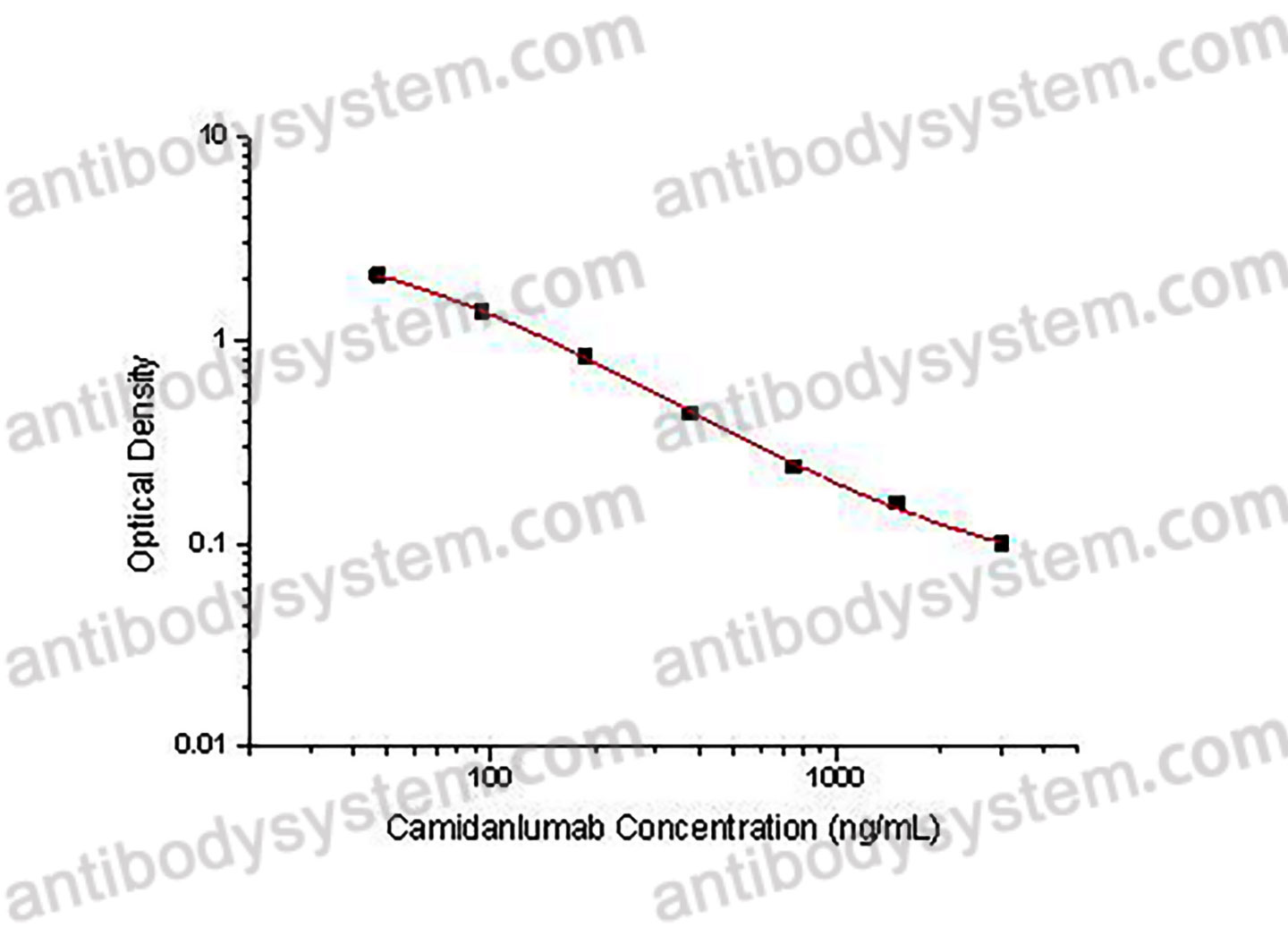
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD25 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Camidanlumab in the sample competitively binds to the pre-coated protein with biotin-labeled Camidanlumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Camidanlumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Camidanlumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
46.88 - 3,000 ng/mL
Sensitivity
35.61 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
1648.9 |
417.1 |
97.3 |
1524.5 |
464.7 |
105.9 |
|
Standard deviation |
123.6 |
22.3 |
9.2 |
90.0 |
25.1 |
8.5 |
|
CV (%) |
7.5 |
5.3 |
9.4 |
5.9 |
5.4 |
8.1 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ADCT-301(unconjugated), HuMax-TAC-ADC, CAS: 921618-45-3
Background
Camidanlumab tesirine is an antibody-drug conjugate against CD25, an antigen expressed in several malignancies, including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). This open-label, dose-escalation and -expansion study (NCT02588092) assessed the safety, activity, pharmacokinetics (PK), and immunogenicity of camidanlumab tesirine in patients with relapsed/refractory ALL/AML. A total of 35 patients (34 AML and 1 ALL) were enrolled and received camidanlumab tesirine intravenously at 3-92 μg/kg once every three weeks (Q3W, n = 26) or 30 or 37.5 μg/kg every week (QW, n = 9). One dose-limiting toxicity of maculopapular rash occurred in the 30 μg/kg QW group; the maximum tolerated dose was not reached. No additional safety concerns or adverse events (AEs) of interest were identified. The most common (>10 % of patients) Grade ≥3 treatment-emergent AEs were febrile neutropenia (25.7 %), lymphopenia, neutropenia, thrombocytopenia or fatigue (all 14.3 %), pneumonia, increased gamma-glutamyltransferase, and hypophosphatemia (each 11.4 %). No signal for serious immune-related AEs such as Guillain-Barré syndrome/polyradiculopathy was observed and there was no evidence of immunogenicity. PK showed rapid clearance with apparent half-life <2 days for conjugated and total antibody, suggesting that Q3W dosing may be insufficient for therapeutic efficacy, and prompting exploration of a QW schedule. Two patients achieved complete responses with incomplete hematologic recovery; one each at 30 and 37.5 μg/kg QW. The trial was terminated during dose escalation due to programmatic reasons other than safety. Hence, recommended dose was not determined.