Catalog No.
KDB86904
Description
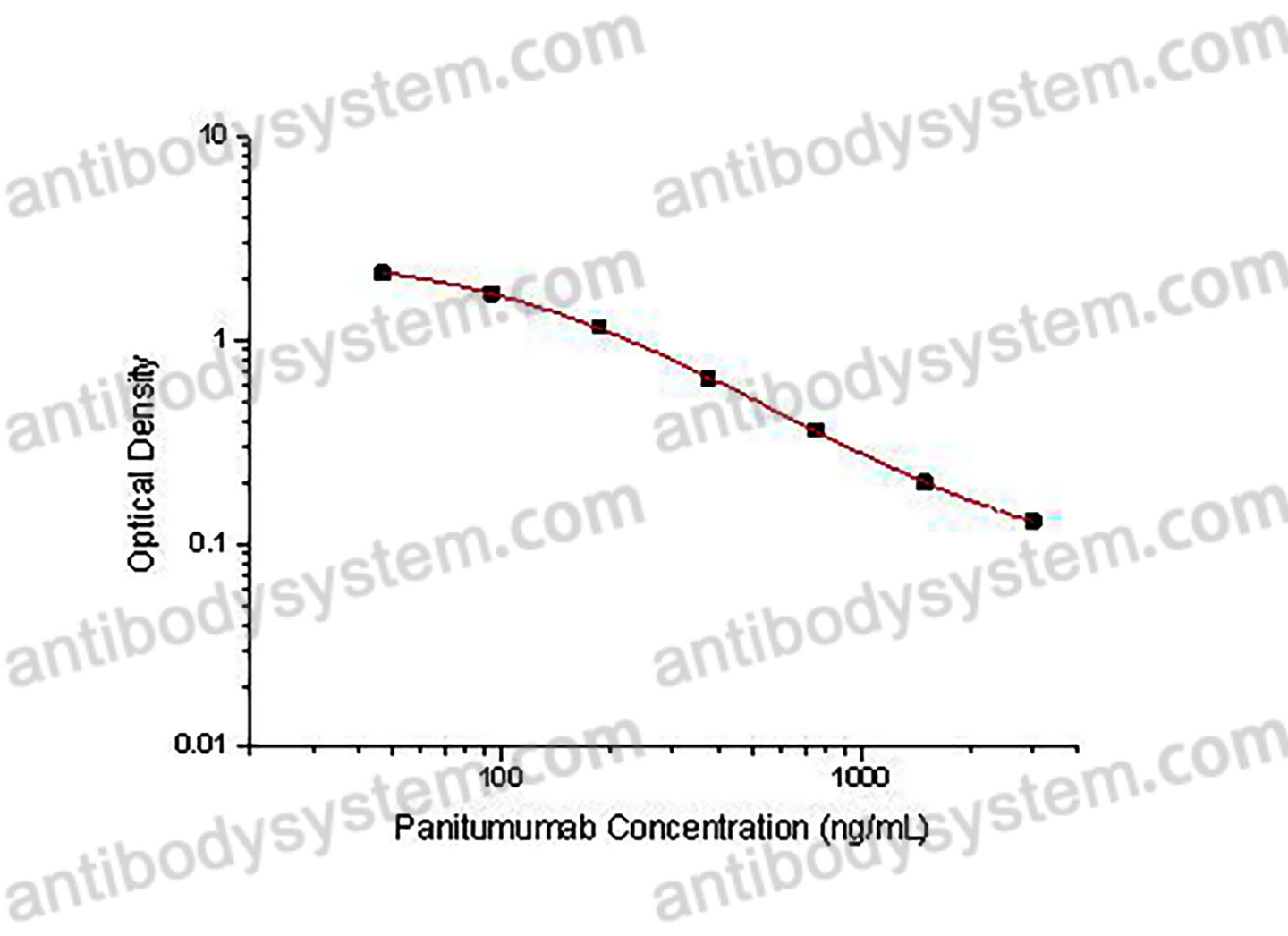
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human EGFR has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Panitumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Panitumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Panitumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Panitumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
46.88 - 3,000 ng/mL
Sensitivity
36.56 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1921.2
|
398.8
|
100.3
|
2337.1
|
422.3
|
77.8
|
|
Standard deviation
|
303.0
|
20.4
|
16.4
|
423.5
|
36.4
|
10.6
|
|
CV (%)
|
15.8
|
5.1
|
16.3
|
18.1
|
8.6
|
13.6
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
ABX-EGF, E7.6.3, CAS: 339177-26-3
Background
Panitumumab, formerly ABX-EGF, is a fully human monoclonal antibody (mAb) specific to the epidermal growth factor receptor (also known as EGF receptor, EGFR, ErbB-1 and HER1 in humans). Panitumumab was originally developed by Abgenix using Abgenix's XenoMouse platform technology, in which engineered mice were utilized to produce human antibodies. This methodology is based on inactivating the mouse immunoglobulin genes that are replaced by a megabase gene containing the human heavy and κ chains. The result is the generation of fully human antibodies that do not contain murine portions of the IgG molecule as chimeric antibodies do. This property avoids the formation of human antimouse antibodies, which may result in more frequent hypersensitivity reactions and shorter half-life.
Identification of Ion Channel-Associated Prognostic Biomarkers for Lung Adenocarcinoma., PMID:40493900
Identifying Molecular Probes for Fluorescence-Guided Surgery in Neuroblastoma: A Systematic Review., PMID:40426729
Exploring Real-World Outcomes of First Line EGFR Inhibitor Use, Cetuximab Versus Panitumumab, in Patients with Left-Sided, RAS Wild-Type, Metastatic Colorectal Cancer., PMID:40413126
Evaluation of the usefulness of protocol-based magnesium supplementation for hypomagnesemia in patients with advanced or recurrent colorectal cancer treated with panitumumab., PMID:40366467
Molecular Characterization and Clinical Outcomes of Pancreatic Neuroendocrine Neoplasms Harboring PAK4-NAMPT Alterations., PMID:40330142
Epidermal growth factor receptor antibody and axial elongation in experimental myopia., PMID:40326259
Construction and evaluation of a novel Stx2a-based immunotoxin against epidermal growth factor receptor (EGFR)., PMID:40315796
Targeting the MAP kinase pathway in colorectal cancer: A journey in personalized medicine., PMID:40310309
KRAS G12C inhibitors as monotherapy or in combination for metastatic colorectal cancer: A proportion and comparative meta-analysis of efficacy and toxicity from phase I-II-III trials., PMID:40274247
Efficacy and safety of oxaliplatin-based chemotherapy as first-line treatment in elderly patients with metastatic colorectal cancer: a meta-analysis., PMID:40260292
Overall Survival Analysis of the Phase III CodeBreaK 300 Study of Sotorasib Plus Panitumumab Versus Investigator's Choice in Chemorefractory KRAS G12C Colorectal Cancer., PMID:40215429
[A Case of Long-Term Prognosis of Advanced Rectosigmoid Cancer with Multiple Metastases Treated with Multidisciplinary Therapy]., PMID:40189763
Black Pigmentation on the Tongue Induced by Long-Term Use of Tetracycline Antibiotics in a Colorectal Cancer Patient With Epidermal Growth Factor Receptor (EGFR) Inhibitor-Associated Skin Lesions: A Case Report and Literature Review., PMID:40130130
Colorectal Cancer: Risk Factors, Novel Approaches in Molecular Screening and Treatment., PMID:40123590
Efficacy and safety of KRAS -G12C inhibitors in colorectal cancer: a systematic review of clinical trials., PMID:40116975
Response to anti-EGFR therapy in chemo-refractory right-sided RAS wild-type metastatic colorectal cancer: a case report and literature review., PMID:40115908
Background Tissue with Native Target Expression Can Determine Presence of Nodal Metastasis in Head and Neck Squamous Cell Carcinoma Patients Infused with Targeted Fluorescent Tracers., PMID:40100567
Treatment of extended RAS/BRAF wild-type metastatic colorectal cancer with anti-EGFR antibody combinations., PMID:40097366
HER2-targeted therapy in colorectal cancer: a comprehensive review., PMID:40087250
The emerging role of Sotorasib plus Panitumumab combination therapy in colorectal cancer treatment., PMID:40080361
Identifying distinct prognostic and predictive contributions of tumor epithelium versus tumor microenvironment in colorectal cancer., PMID:40075322
Costs of Adverse Event Management Associated with First-Line Cetuximab or Panitumumab in Metastatic Colorectal Cancer Patients in Saudi Arabia., PMID:40061835
Molecular Markers of Occult Lymph Node Metastasis in Head and Neck Squamous Cell Carcinoma (HNSCC) Patients., PMID:40018925
Aphthous-Like Stomatitis in a Patient Receiving Panitumumab., PMID:40017685
The Pharmacologic Inhibition of KRAS Mutants as a Treatment for Cancer: Therapeutic Principles and Clinical Results., PMID:40009739
Real-World Evidence of Bevacizumab and Panitumumab Drug Resistance and Drug Ineffectiveness from EudraVigilance Database., PMID:40002260
[A Case of Colorectal Cancer with Multiple Unresectable Liver Metastases That Achieved R0 after Chemotherapy and Laparoscopic Two-Stage Hepatectomy]., PMID:39949006
Could Panitumumab with very low dose Capecitabine be an option as a maintenance regimen., PMID:39945465
Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C., PMID:39938112
Results of the Phase I/II Study and Preliminary B-cell Gene Signature of Combined Inhibition of Glutamine Metabolism and EGFR in Colorectal Cancer., PMID:39927885
Using methods to extend inferences to specific target populations to improve the precision of subgroup analyses., PMID:39924128
Monitoring of occupational exposure to hazardous medicinal products in robotic compounding., PMID:39890433
Beyond chemotherapy: Exploring 5-FU resistance and stemness in colorectal cancer., PMID:39863147
An Exosome-Based Liquid Biopsy Predicts Depth of Response and Survival Outcomes to Cetuximab and Panitumumab in Metastatic Colorectal Cancer: The EXONERATE Study., PMID:39820673
Neo-adjuvant FOLFOX with and without panitumumab for patients with KRAS-wt locally advanced colon cancer: results following an extended biomarker panel on the FOxTROT trial embedded phase II population., PMID:39805350
Panitumumab plus 5-fluorouracil and folinic acid or 5-fluorouracil and folinic acid alone as maintenance therapy in RAS wild-type metastatic colorectal cancer (PanaMa, AIO KRK 0212): final efficacy analysis of a randomised, open-label, phase 2 trial., PMID:39802302
Panitumumab versus cetuximab in combination with irinotecan in refractory metastatic colorectal cancer., PMID:39799855
AGITG MONARCC: A Randomized Phase 2 Study of Panitumumab Monotherapy and Panitumumab Plus 5-Fluorouracil as First-Line Therapy for Older Patients With RAS and BRAF Wild Type Metastatic Colorectal Cancer. A Study by the Australasian Gastro-Intestinal Trials Group (AGITG)., PMID:39779412
Au@109Pd Core-Shell Nanoparticles Conjugated to Panitumumab for the Combined β--Auger Electron Therapy of Triple-Negative Breast Cancer., PMID:39769315
Development of KRAS Inhibitors and Their Role for Metastatic Colorectal Cancer., PMID:39752885
Limited Efficacy of Anti-EGFR Monoclonal Antibodies in Colorectal Cancer Patients with Rare RAS Variants: Analysis of the C-CAT Database., PMID:39727997
[Case of Minocycline-Induced Thrombocytopenia during Chemotherapy for Ascending Colon Cancer]., PMID:39721793
The efficacy of targeted therapy and/or immunotherapy with or without chemotherapy in patients with colorectal cancer: A network meta-analysis., PMID:39716565
Second-line systemic treatment for metastatic colorectal cancer: A systematic review and Bayesian network meta-analysis based on RCT., PMID:39715232
Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment., PMID:39682164
Prognostic value of radiologic and pathological response in colorectal cancer liver metastases upon systemic induction treatment: subgroup analysis of the CAIRO5 trial., PMID:39667310
Intensified Total Neoadjuvant Therapy in Patients With Locally Advanced Rectal Cancer: Long-term Results of a Prospective Phase II Study., PMID:39667262
Post-marketing safety of panitumumab: a real-world pharmacovigilance study., PMID:39651795
The Use of Fluorescent Markers to Detect and Delineate Head and Neck Cancer: A Scoping Review., PMID:39629534
Effective Management of Severe Epidermal Growth Factor Receptor Inhibitors-Induced Papulopustular Eruption With Low-Dose Isotretinoin and Corticosteroids., PMID:39624566