Catalog No.
KDA29105
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD262 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Tigatuzumab in the sample competitively binds to the pre-coated protein with biotin-labeled Tigatuzumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Tigatuzumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
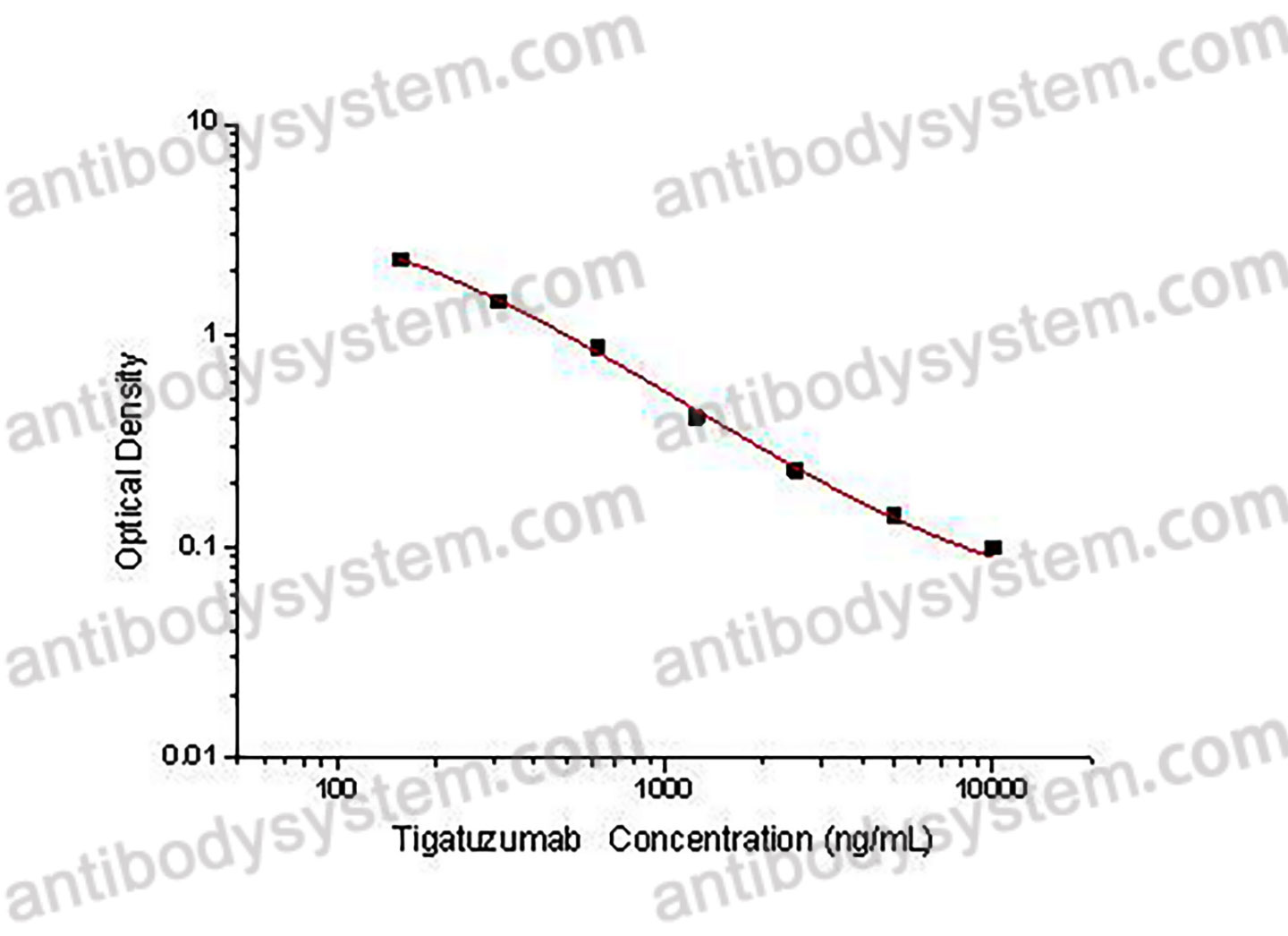
Used for the quantitative determination of Tigatuzumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
156.25 - 10,000 ng/mL
Sensitivity
96.15 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
5428.6 |
1499.4 |
580.8 |
6262.7 |
1647.2 |
629.8 |
|
Standard deviation |
1012.6 |
96.1 |
43.7 |
778.3 |
213.4 |
77.9 |
|
CV (%) |
18.7 |
6.4 |
7.5 |
12.4 |
13.0 |
12.4 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CS-1008, TRA-8, CAS: 918127-53-4
Background
Tigatuzumab is the humanized version of the agonistic murine monoclonal antibody TRA-8 that binds to the death receptor 5 and induces apoptosis of human cancer cell lines via the caspase cascade. The combination of tigatuzumab and gemcitabine inhibits tumor growth in murine pancreatic xenografts. This phase 2 trial evaluated the efficacy of tigatuzumab combined with gemcitabine in 62 chemotherapy-naive patients with histologically or cytologically confirmed unresectable or metastatic pancreatic cancer. Patients received intravenous tigatuzumab (8 mg/kg loading dose followed by 3 mg/kg weekly) and gemcitabine (1000 mg/m(2) once weekly for 3 weeks followed by 1 week of rest) until progressive disease (PD) or unacceptable toxicity occurred. The primary end point was progression-free survival (PFS) at 16 weeks. Secondary end points included objective response rate (ORR) (complete responses plus partial responses), duration of response, and overall survival (OS). Safety of the combination was also evaluated. Mean duration of treatment was 18.48 weeks for tigatuzumab and 17.73 weeks for gemcitabine. The PFS rate at 16 weeks was 52.5% (95% confidence interval [CI], 39.3-64.1%). The ORR was 13.1%; 28 (45.9%) patients had stable disease and 14 (23%) patients had PD. Median PFS was 3.9 months (95% CI, 2.2-5.4 months). Median OS was 8.2 months (95% CI, 5.1-9.6 months). The most common adverse events related to tigatuzumab were nausea (35.5%), fatigue (32.3%), and peripheral edema (19.4%). Tigatuzumab combined with gemcitabine was well tolerated and may be clinically active for the treatment of chemotherapy-naive patients with unresectable or metastatic pancreatic cancer.