Catalog No.
KDA29103
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD262 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Drozitumab in the sample competitively binds to the pre-coated protein with biotin-labeled Drozitumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Drozitumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Drozitumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
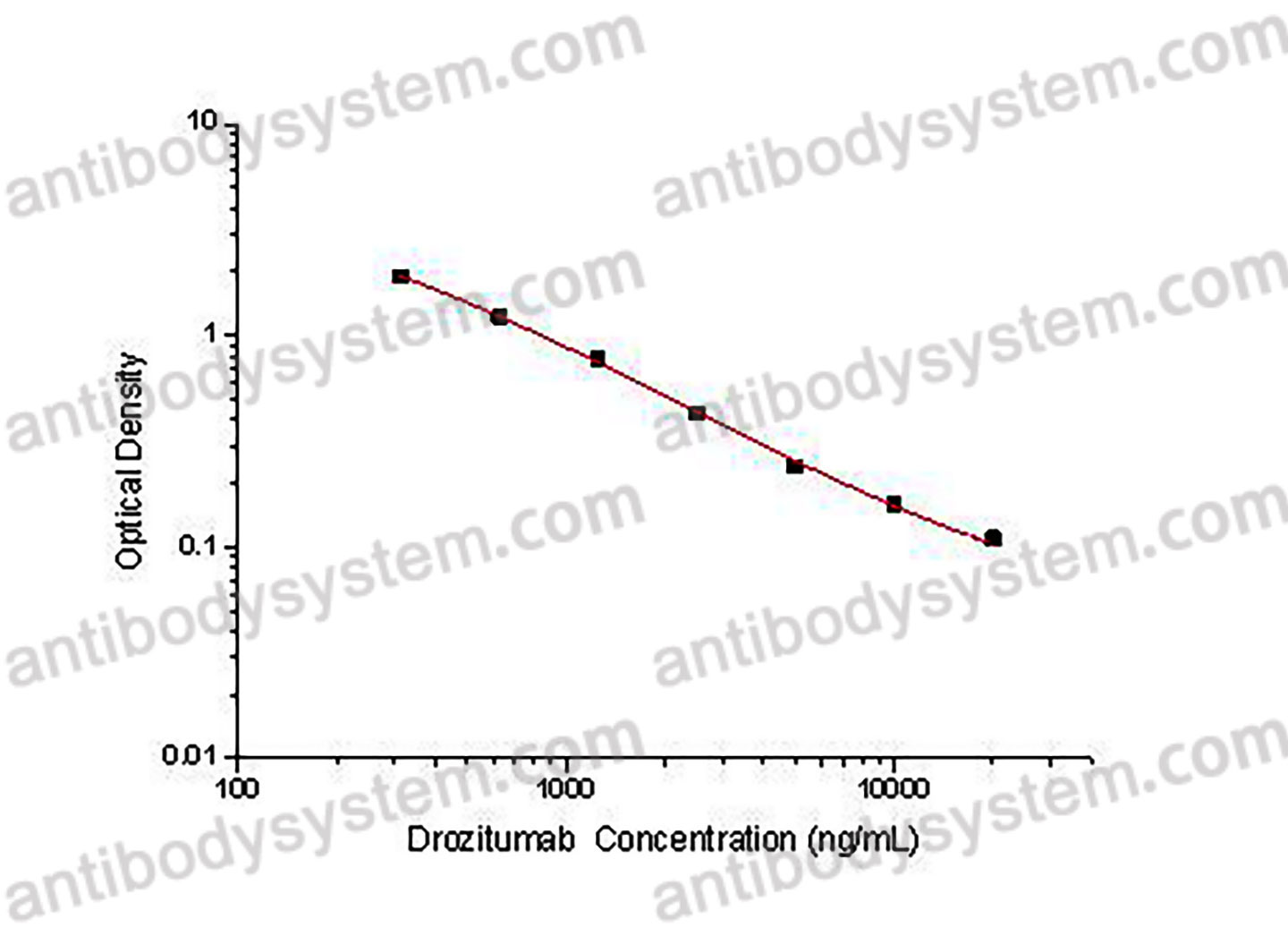
Range
312.5 - 20,000 ng/mL
Sensitivity
114.28 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
10325.7 |
2732.3 |
1049.9 |
12014.0 |
2897.0 |
1062.2 |
|
Standard deviation |
1075.6 |
89.9 |
53.7 |
2231.1 |
373.1 |
128.7 |
|
CV (%) |
10.4 |
3.3 |
5.1 |
18.6 |
12.9 |
12.1 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
PRO95780, anti-DR5, rhuMAb DR5, CAS: 912628-39-8
Background
Drozitumab is a human monoclonal antibody directed against tumor necrosis factor receptor superfamily member 10B (TNFRSF10B), a member of tumor necrosis factor related apoptosis-inducing ligand (TRAIL). It was developed by Genentech as a drug for the treatment of cancers. This drug has been investigating in various trials for treating cancers including chondrosarcoma, colorectal cancer, non-Hodgkin lymphoma, and non-small cell lung cancer. The efficacy and selectivity of drozitumab in rhabdomyosarcoma (RMS) has been evaluated in preclinical models. And results showed that drozitumab is effective, in vitro, against the majority of RMS cell lines that express caspase-8 and, in vivo, may provide long-term control of RMS. In preclinical glioblastoma models, drozitumab has been found to induce apoptosis and colony formation in several glioblastoma cell lines combined with BV6 (combination index<0.1). A study about triple-negative breast cancer reported that drozitumab could induce apoptosis in mesenchymal TNBC cell lines but not in cell lines from other breast cancer subtypes. The previous study about the treatment of pancreatic ductal adenocarcinoma has revealed that drozitumab could selectively eliminate CSCs, resulting in tumor growth inhibition and even regression of pancreatic tumors.