Catalog No.
KDA29102
Description
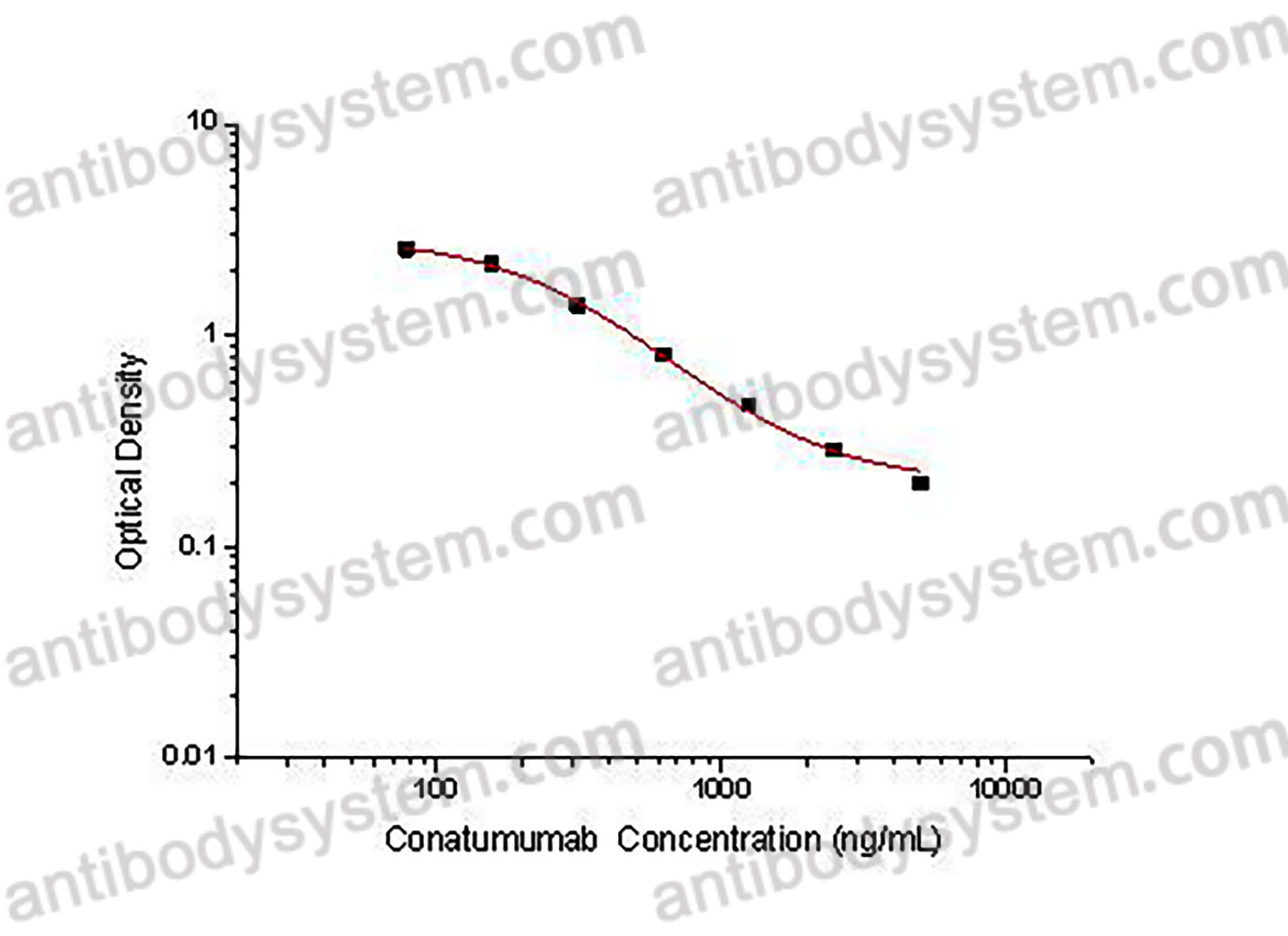
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human CD262 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Conatumumab in the sample competitively binds to the pre-coated protein with biotin-labeled Conatumumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Conatumumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Conatumumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
78.13 - 5,000 ng/mL
Sensitivity
91.95 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2786.1
|
664.0
|
174.6
|
2871.2
|
689.7
|
144.4
|
|
Standard deviation
|
316.0
|
54.2
|
28.8
|
334.6
|
39.5
|
19.0
|
|
CV (%)
|
11.3
|
8.2
|
16.5
|
11.7
|
5.7
|
13.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
AMG 655, TRAIL-R2mAb, XG1-048 v w, CAS: 896731-82-1
Background
Conatumumab (previously called AMG-655) is a fully human Immunoglobulin G1 (IgG1) type monoclonal binding to tumor necrosis factor receptor superfamily member 10B (TNFRSF10B) which could induce apoptosis in many types of human cancer cells. This drug was developed by Amgen Inc and Japanese licensee Takeda Bio Development Center Ltd with potential antineoplastic activity. This drug has been investigated in trials studying the treatment of sarcoma, lymphoma, oncology, colon cancer, and rectal cancer. Conatumumab can elicit the apoptosis in cell lines derived from colon and pancreatic cancers, as well as in mice bearing xenograft tumors in some in vitro and in vivo assays. Clinical trials in phase I has assessed the safety of conatumumab as a monotherapy as well as in combination with other antibody therapies or standard chemotherapeutic regimes. And the anti-conatumumab antibody responses have not been observed in previous trials. In addition, conatumumab has been found to enhance the antitumor activity of agents like irinotecan and gemcitabine in some preclinical researches. A trail about conatumumab combined with AMG 479 has shown that it is well-tolerated and no drug-drug interactions and the phase II development of this combination is ongoing.
The generation of stable microvessels in ischemia is mediated by endothelial cell derived TRAIL., PMID:39365855
TRAIL-Based Therapies Efficacy in Pediatric Bone Tumors Models Is Modulated by TRAIL Non-Apoptotic Pathway Activation via RIPK1 Recruitment., PMID:36428719
Combined and targeted drugs delivery system for colorectal cancer treatment: Conatumumab decorated, reactive oxygen species sensitive irinotecan prodrug and quercetin co-loaded nanostructured lipid carriers., PMID:35049388
Durable Complete Response to AMG 655 (Conatumumab) and Vorinostat in a Patient With Relapsed Classical Hodgkin Lymphoma: Extraordinary Response from a Phase 1b Clinical Protocol., PMID:32828719
Efficacy and Safety of Regorafenib in Combination with Chemotherapy as Second-Line Treatment in Patients with Metastatic Colorectal Cancer: A Network Meta-Analysis and Systematic Literature Review., PMID:32770529
The serum protein transthyretin as a platform for dimerization and tetramerization of antibodies and Fab fragments to enable target clustering., PMID:32518163
[99mTc]duramycin for cell death imaging: Impact of kit formulation, purification and species difference., PMID:29031229
Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations., PMID:28675660
99mTc-Duramycin SPECT Imaging of Early Tumor Response to Targeted Therapy: A Comparison with 18F-FDG PET., PMID:27879368
A network meta-analysis on the efficacy of sixteen targeted drugs in combination with chemotherapy for treatment of advanced/metastatic colorectal cancer., PMID:27806321
Onto better TRAILs for cancer treatment., PMID:26943322
Caspase-8 expression is predictive of tumour response to death receptor 5 agonist antibody in Ewing's sarcoma., PMID:26291055
Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity., PMID:25043603
TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells., PMID:24909167
Anticancer activity of the type I insulin-like growth factor receptor antagonist, ganitumab, in combination with the death receptor 5 agonist, conatumumab., PMID:24816908
TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial., PMID:24122767
Benzazepinones and benzoxazepinones as antagonists of inhibitor of apoptosis proteins (IAPs) selective for the second baculovirus IAP repeat (BIR2) domain., PMID:24083782
Death receptors as targets in cancer., PMID:23638798
A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer., PMID:23510984
A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer., PMID:23370314
Intracellular-signaling tumor-regression modeling of the pro-apoptotic receptor agonists dulanermin and conatumumab., PMID:22932917
Therapeutic targeting of the TNF superfamily: a promising treatment for advanced endometrial adenocarcinoma., PMID:22885470
A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer., PMID:22700995
Validity of the FACT Hepatobiliary (FACT-Hep) questionnaire for assessing disease-related symptoms and health-related quality of life in patients with metastatic pancreatic cancer., PMID:22678353
First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study., PMID:22240283
Implementation of design of experiments (DOE) in the development and validation of a cell-based bioassay for the detection of anti-drug neutralizing antibodies in human serum., PMID:22119514
Conatumumab: a novel monoclonal antibody against death receptor 5 for the treatment of advanced malignancies in adults., PMID:21877997
Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery., PMID:21875750
Leveraging image cytometry for the development of clinically feasible biomarkers: evaluation of activated caspase-3 in fine needle aspirate biopsies., PMID:21704844
Characterization of 64Cu-DOTA-conatumumab: a PET tracer for in vivo imaging of death receptor 5., PMID:21571804
Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors., PMID:21161528
A first-in-human study of conatumumab in adult patients with advanced solid tumors., PMID:20947515
Measurement of conatumumab-induced apoptotic activity in tumors by fine needle aspirate sampling., PMID:20623688
Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer., PMID:20496264
Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types., PMID:20150762
American Society of Hematology--51st annual meeting & exposition. Part 1., PMID:20127549
Gateways to clinical trials., PMID:19798455