Catalog No.
DHA30605
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-Lambda
Clonality
Monoclonal
Target
GDF-8, GDF8, MSTN, Myostatin, Growth/differentiation factor 8
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
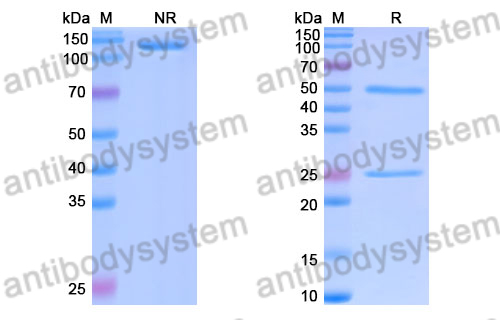
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
O14793
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
SRK 015, SRK-015, SRK015, CAS: 2278276-46-1
Clone ID
Apitegromab
Long-term efficacy, safety, and patient-reported outcomes of apitegromab in patients with spinal muscular atrophy: results from the 36-month TOPAZ study., PMID:39105058
Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study., PMID:38830133
Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study., PMID:38330285
Advances and limitations for the treatment of spinal muscular atrophy., PMID:36329412
Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases., PMID:35727341
Preclinical Safety Assessment and Toxicokinetics of Apitegromab, an Antibody Targeting Proforms of Myostatin for the Treatment of Muscle-Atrophying Disease., PMID:34255983
A Randomized Phase 1 Safety, Pharmacokinetic and Pharmacodynamic Study of the Novel Myostatin Inhibitor Apitegromab (SRK-015): A Potential Treatment for Spinal Muscular Atrophy., PMID:33963971