Catalog No.
DHJ29001
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CLEC4C, C-type lectin superfamily member 7, CLECSF11, C-type lectin domain family 4 member C, Dendritic lectin, CD303, DLEC, HECL, Blood dendritic cell antigen 2, BDCA-2, BDCA2, CLECSF7
Concentration
3.45 mg/ml
Endotoxin level
Please contact with the lab for this information.
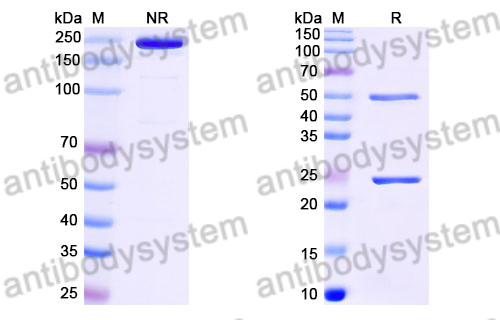
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q8WTT0
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BIIB 059, BIIB-059, BIIB059, CAS: 2407378-48-5
Clone ID
Litifilimab
Pharmacodynamic Effects of Litifilimab in Lupus in the LILAC Phase II Study: Rapid and Sustained Reductions in Type I Interferon-Associated Gene Expression and Cytokines., PMID:40488251
An Integrative Mechanistic Model of Type 1 IFN-Mediated Inflammation in Systemic Lupus Erythematosus., PMID:40364448
RF - New Treatments in Cutaneous Lupus Erythematosus: Current and Future Perspectives., PMID:40204141
A focused report on IFN-1 targeted therapies for lupus erythematosus., PMID:40047795
Part B of the LILAC study of litifilimab for cutaneous lupus erythematosus: a plain language summary., PMID:39995069
Part A of the LILAC study of litifilimab for systemic lupus erythematosus: a plain language summary., PMID:39995062
Evolving Treatment Strategies for Systemic Lupus Erythematosus in Clinical Practice: A Narrative Review., PMID:39759646
Deucravacitinib shows superior efficacy and safety in cutaneous lupus erythematosus compared to various biologics and small molecules - A systematic review and meta-analysis., PMID:39694128
An update on clinical trials for cutaneous lupus erythematosus., PMID:38491743
Emerging biologic therapies for systemic lupus erythematosus., PMID:38299618
The development of litifilimab (BIIB 059) for cutaneous and systemic lupus erythematosus., PMID:37877249
Emerging immunotherapeutic strategies for cutaneous lupus erythematosus: an overview of recent phase 2 and 3 clinical trials., PMID:37860982
Litifilimab (BIIB059), a promising investigational drug for cutaneous lupus erythematosus., PMID:37148249
Cutaneous Lupus Erythematosus: An Update on Pathogenesis and Future Therapeutic Directions., PMID:37140884
Trial of Anti-BDCA2 Antibody Litifilimab for Systemic Lupus Erythematosus., PMID:36069871
Trial of Anti-BDCA2 Antibody Litifilimab for Cutaneous Lupus Erythematosus., PMID:35939578