Clinical Features of Children With MOG-IgG Who Fulfill Criteria of Multiple Sclerosis and Overlapping Disorders., PMID:40505071
The use of Bevacizumab in the treatment of brain arteriovenous malformations: a systematic review., PMID:40504282
IFITM3 deficient mice as a model for testing influenza virus vaccines., PMID:40501891
Retrospective cohort study of anti-CGRP monoclonal antibody unresponsive migraine individuals treated with atogepant: The RESCUE study., PMID:40501126
Impact of Universal Screening for HDV in HBV-Infected Patients on Chronic HDV Detection Rate in Israel., PMID:40497662
Seroprevalence of tick-borne infections in blood donors in Europe: a systematic review., PMID:40497133
Anti-NMDAR Encephalitis With Serial Negative MRI Findings: An Evaluation Using Autoimmune Psychosis Criteria., PMID:40496240
Induction With Antithymocyte Globulin Is Associated With Decreased Mortality and PTLD in Pediatric Liver Transplantation: A UNOS Data Analysis., PMID:40490923
Eliminating The Need for Sequential Confirmation of Response in Multiple Myeloma., PMID:40489635
Impact of sirtuin‑1 expression on progression‑free survival in non‑endometrioid endometrial cancer: A retrospective cohort study., PMID:40486084
Certolizumab pegol to prevent adverse pregnancy outcomes in patients with antiphospholipid syndrome and lupus anticoagulant (IMPACT): results of a prospective, single-arm, open-label, phase 2 trial., PMID:40483169
Tumefactive demyelination as the first presentation of MOG ab-associated disease., PMID:40479755
Surgical considerations for tumour-infiltrating lymphocyte therapy in melanoma: results from a randomized phase III trial., PMID:40474841
Utilization of anti-CD20 antibodies for treatment of childhood nephrotic syndrome, 2010 to 2022., PMID:40473981
Burden of illness among patients with relapsed or refractory multiple myeloma, and eligible for B-cell maturation antigen-targeted therapies., PMID:40471555
A Phase I Study of the Anti-CEACAM6 Antibody Tinurilimab (BAY 1834942) in Patients with Advanced Solid Tumors., PMID:40465170
Safety, Tolerability, and Immunogenicity of mRNA-1345 in Adults at Increased Risk for RSV Disease Aged 18 to 59 Years., PMID:40464662
Effects of cottonseed meal bioactive peptides on growth performance, ileal digestibility, serum amino acid and immune responses of broiler chickens., PMID:40464591
Humoral immune response following the third dose of BNT162b2 received by employees at a Slovak cancer healthcare facility., PMID:40463399
Analytical treatment interruption among women with HIV in southern Africa who received VRC01 or placebo in the Antibody Mediated Prevention Study: ATI stakeholder engagement, implementation and early clinical data., PMID:40462491
Association of cancer and anti-synthetase syndrome: a retrospective multicentre study., PMID:40460913
Genetics in a Danish Common Variable Immunodeficiency Cohort., PMID:40455168
Comparative effectiveness of natalizumab and anti-CD20 monoclonal antibodies in relapsing-remitting multiple sclerosis: a real-world propensity-score matched study., PMID:40451284
Sasanlimab plus BCG in BCG-naive, high-risk non-muscle invasive bladder cancer: the randomized phase 3 CREST trial., PMID:40450141
Anlotinib plus toripalimab as a first-line treatment in patients with advanced gastric cancer and performance status 2: the phase II APICAL-GC trial., PMID:40450051
Patritumab deruxtecan in leptomeningeal metastatic disease of solid tumors: the phase 2 TUXEDO-3 trial., PMID:40447851
Intracranial and systemic progression on amivantamab in platinum-treated epidermal growth factor receptor exon 20 insertion-mutated advanced non-small cell lung cancer., PMID:40446773
The recombinant anti-MET/EpCAM bispecific antibody fragment: a promising novel therapeutic approach for breast cancer treatment., PMID:40445574
Clinical spectrum and long-term outcomes of antibody-negative severe autoimmune encephalitis: a retrospective study., PMID:40443652
Adverse Cutaneous Reactions to Monoclonal Antibodies: Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis, Erythema Multiforme, and Fixed Drug Eruption - A Systematic Review., PMID:40443459
Serum antineuronal antibodies in patients with post-COVID-19 condition - association to intensive care., PMID:40441540
PD-L1 Expression in Biliary Tract Cancer: Comparison Across Antibody Clones and Role as a Predictor of Response to Chemoimmunotherapy: A Meta-Analysis., PMID:40440581
Expression of Membrane Targets for Therapeutics in RET-Positive Non-Small Cell Lung Cancer., PMID:40440007
Durable complete response in leptomeningeal disease of EGFR mutated non-small cell lung cancer to amivantamab, an EGFR-MET receptor bispecific antibody, after progressing on osimertinib., PMID:40439479
Incidence of catheter-related bloodstream infection (CRBSI) in immunosuppressed hosts post solid organ transplant (SOT): a single center experience., PMID:40438131
Latin American RAND/UCLA modified Delphi consensus recommendations for management and treatment of adult MOGAD patients in clinical practice., PMID:40435656
The Safety and Immunogenicity of a Quadrivalent Influenza Subunit Vaccine in Healthy Children Aged 6-35 Months: A Randomized, Blinded and Positive-Controlled Phase III Clinical Trial., PMID:40432079
Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials., PMID:40430899
Q Fever-Related Community Infections: United States Exposure to Coxiella burnetii., PMID:40430780
Cellular Metabolic Disorders in a Cohort of Patients with Sjogren's Disease., PMID:40429812
High Rate of Seroprotection With Heplisav-B in Patients With Inflammatory Bowel Disease., PMID:40424081
Programmed death-ligand 1 tumor proportion score in predicting the safety and efficacy of PD-1/PD-L1 antibody-based therapy in patients with advanced non-small cell lung cancer: A retrospective, multicenter, observational study., PMID:40413619
Systematic Literature Review of DOACs as Treatment for Confirmed or Suspected Heparin-Induced Thrombocytopenia (HIT)., PMID:40411430
Safety and Efficacy of Pemivibart, a Long-Acting Monoclonal Antibody, for Prevention of Symptomatic COVID-19: Interim Results From a Phase 3 Randomized Clinical Trial (CANOPY)., PMID:40410927
A highly specific antibody profiled by hapten prediction and its application in an immunoassay for Anilazine., PMID:40408967
Evaluation of Anti-SARS-CoV-2 IgG Responses in a Clinical Study of a Biosimilar Candidate to Denosumab Using Singlicate Analysis., PMID:40408051
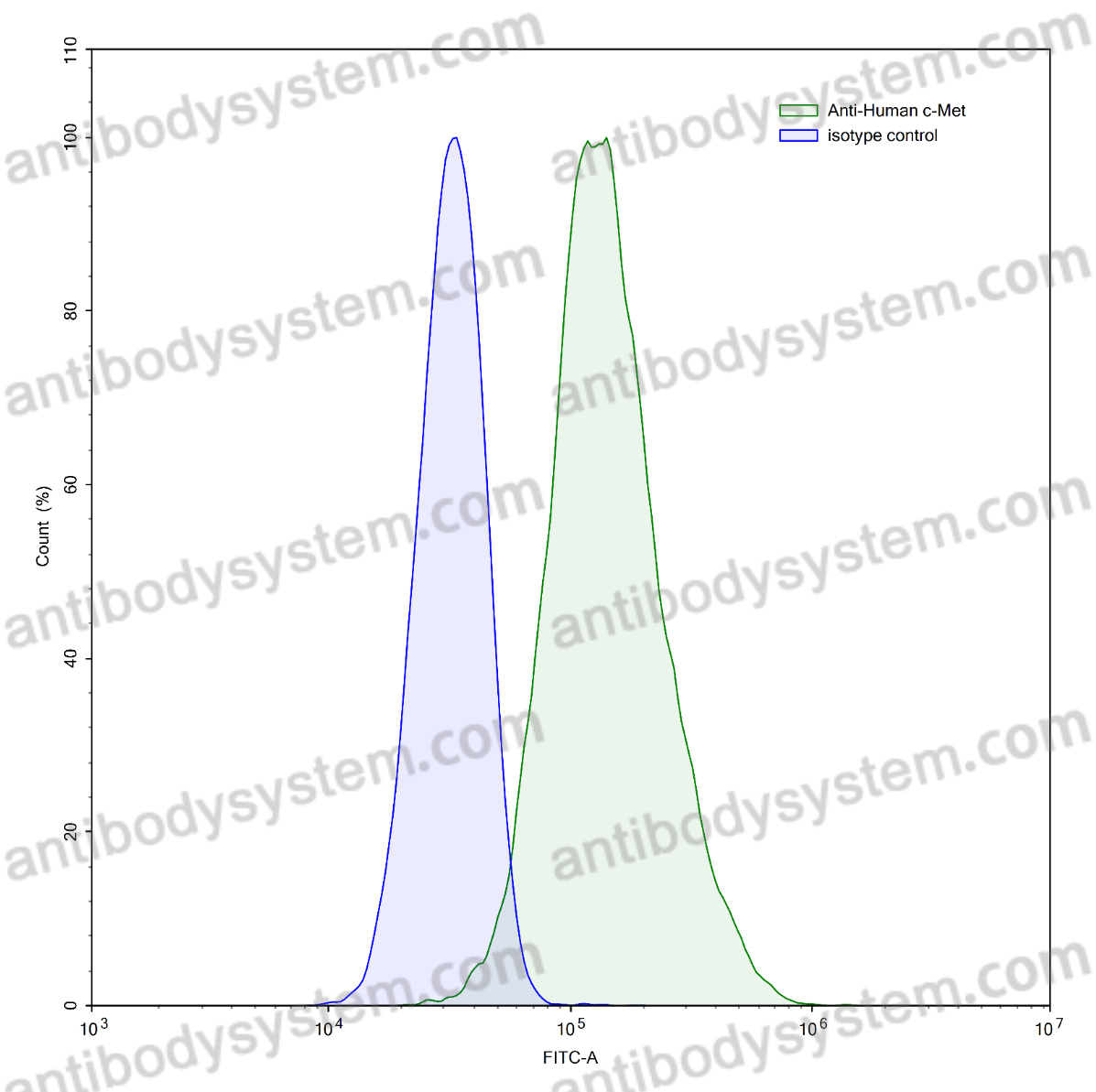
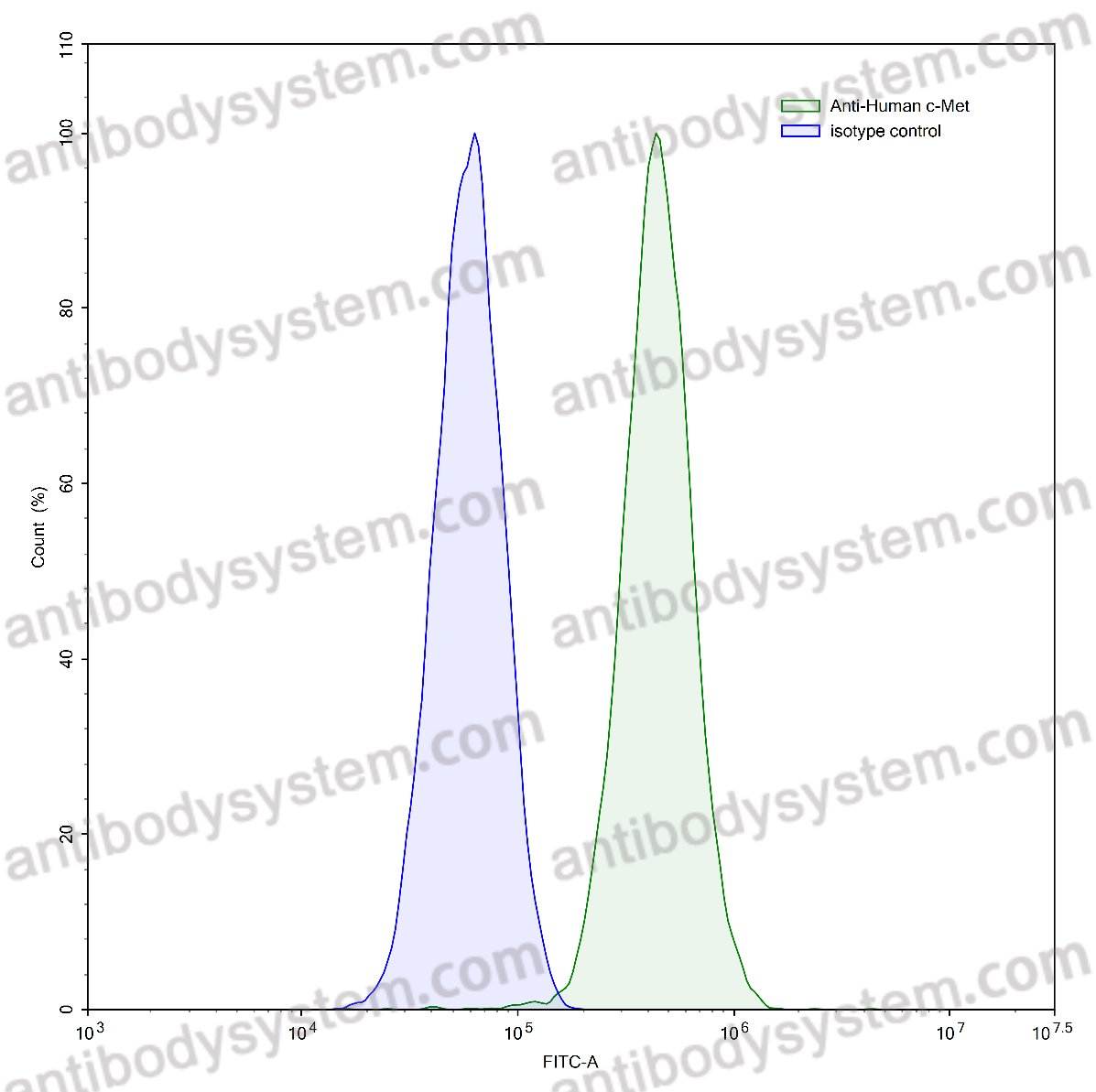
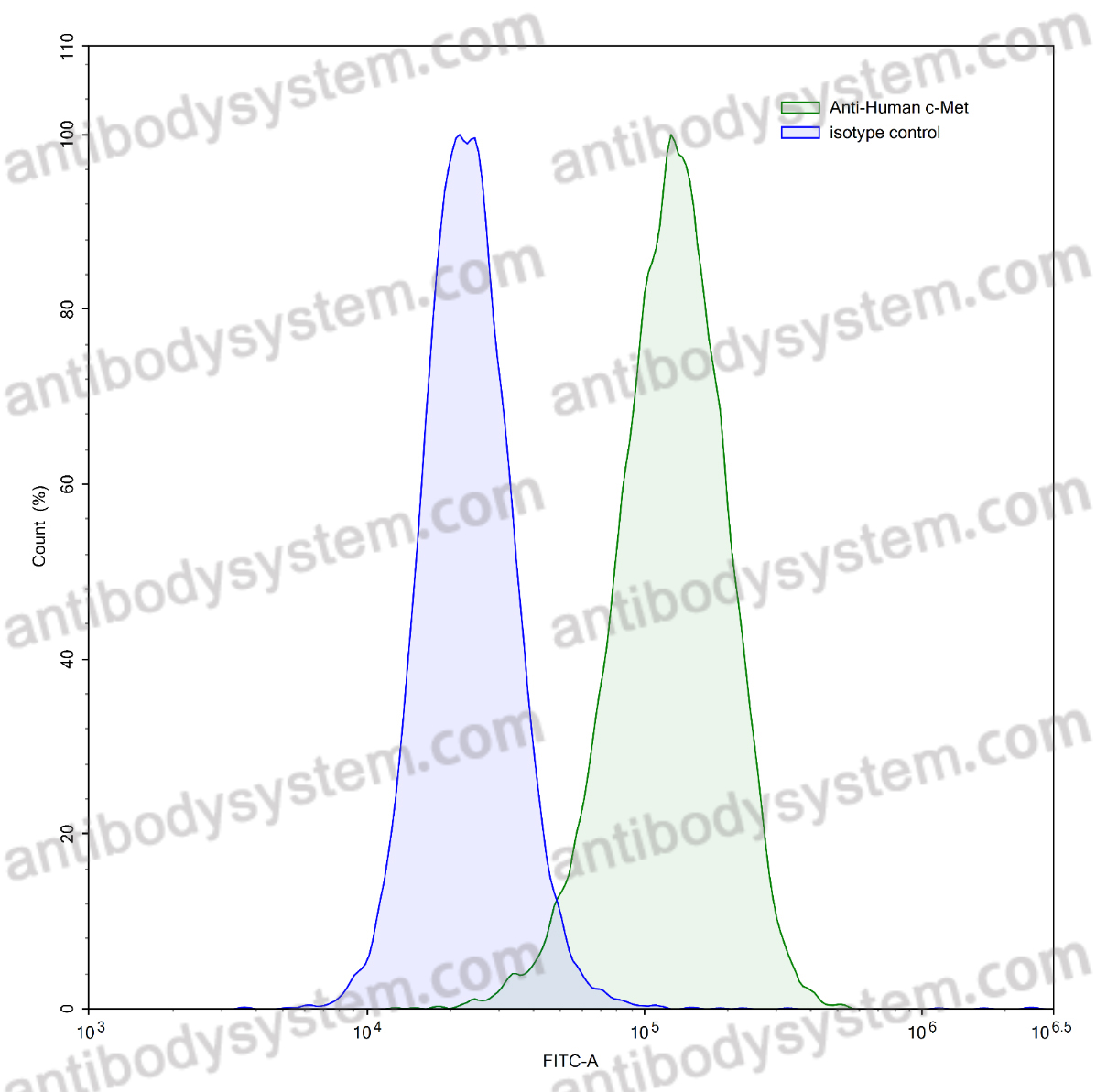
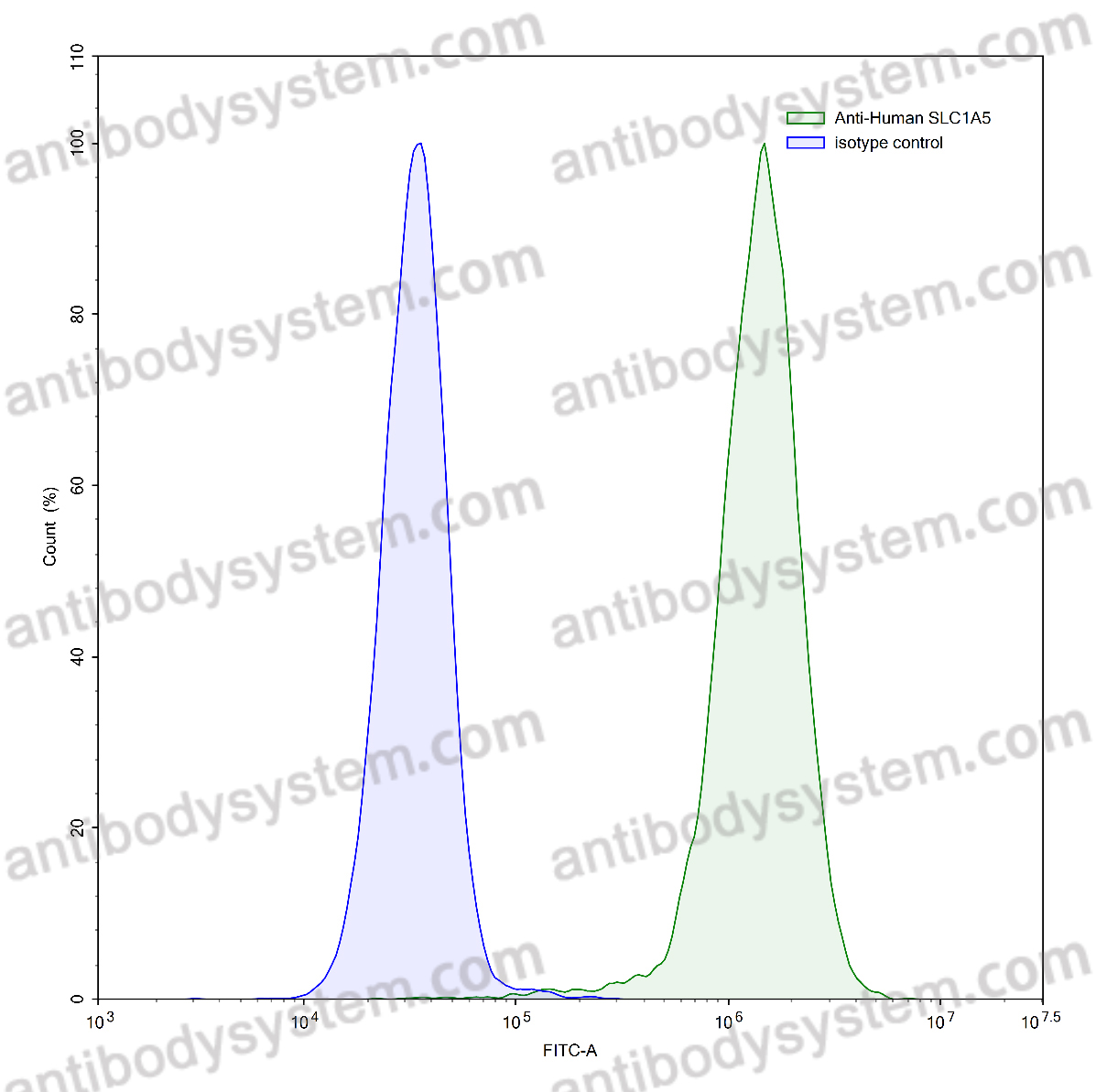
Design of heavy chain antibody-drug conjugates targeting c-Met to eradicate lung adenocarcinoma giant tumors with a single-dose., PMID:40404557
Unilateral Graves' disease: a case report with concomitant thyroid cancer and systematic review of literature., PMID:40401585
Unique characteristics of autoantibodies targeting MET in patients with breast and lung cancer., PMID:40401526
Psychometric validation of the EORTC QLQ-OES18 in patients with advanced or metastatic esophageal squamous cell carcinoma., PMID:40397297