Catalog No.
DHJ70117
Description
Socazolimab is an anti-PD-L1 antibody licensed from Sorrento for the Greater China Territory by Lee’s Pharma.
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-lambda2
Clonality
Monoclonal
Target
B7-H1, Programmed cell death 1 ligand 1, PDCD1 ligand 1, PDCD1L1, B7 homolog 1, PDCD1LG1, PDL1, hPD-L1, Programmed death ligand 1, B7H1, PD-L1, CD274
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
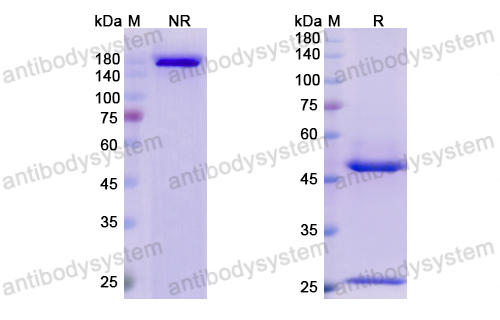
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9NZQ7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
STI-1014, STI-A1014, ZKAB-001, ZKAB001 CAS: 2305043-30-3
Clone ID
Socazolimab
Promising outcomes of the PD-L1 inhibitor Socazolimab in recurrent and metastatic cervical cancer: a case report., PMID:40206580
A multicenter, randomized, double-blind, placebo-controlled phase 3 study of Socazolimab or placebo combined with carboplatin and etoposide in the first-line treatment of extensive-stage small cell lung cancer., PMID:39800716
Phase Ib study of anti-PD-L1 monoclonal antibody socazolimab in combination with nab-paclitaxel as first-line therapy for advanced urothelial carcinoma., PMID:39418340
Efficacy and Safety of Anti-Programmed Death-Ligand 1 Monoclonal Antibody Socazolimab With Carboplatin and Etoposide for Extensive-Stage SCLC: Results From the Phase 1b Clinical Trial., PMID:37020926
Comparing a PD-L1 inhibitor plus chemotherapy to chemotherapy alone in neoadjuvant therapy for locally advanced ESCC: a randomized Phase II clinical trial : A randomized clinical trial of neoadjuvant therapy for ESCC., PMID:36882775
Efficacy and Safety of the Anti-PD-L1 mAb Socazolimab for Recurrent or Metastatic Cervical Cancer: a Phase I Dose-Escalation and Expansion Study., PMID:36136294