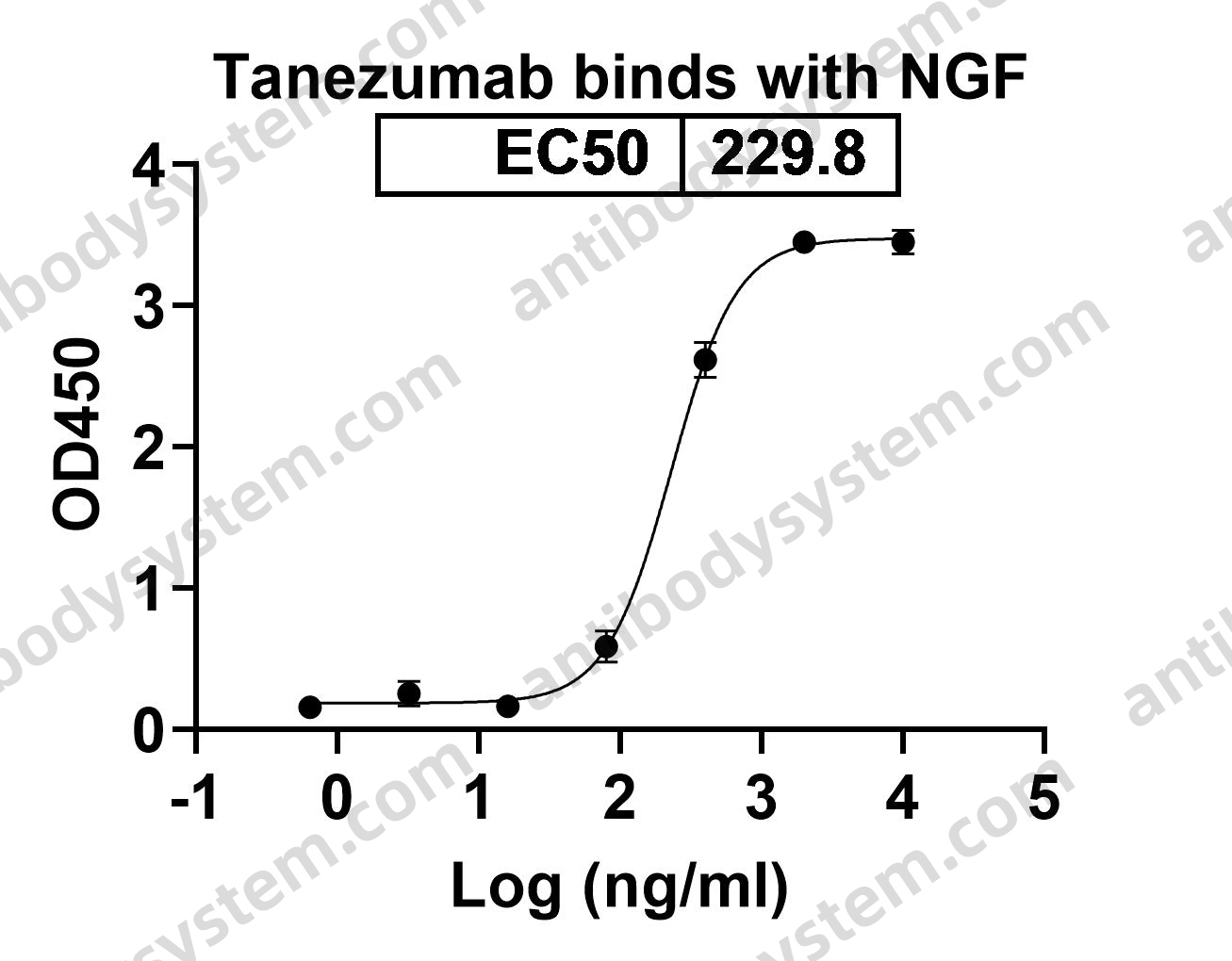
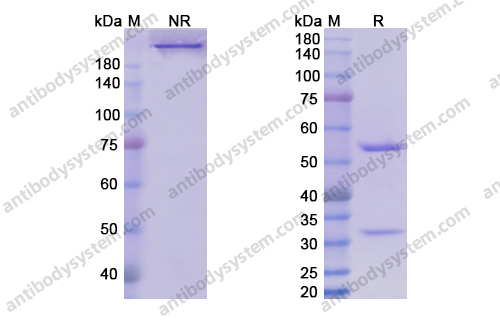
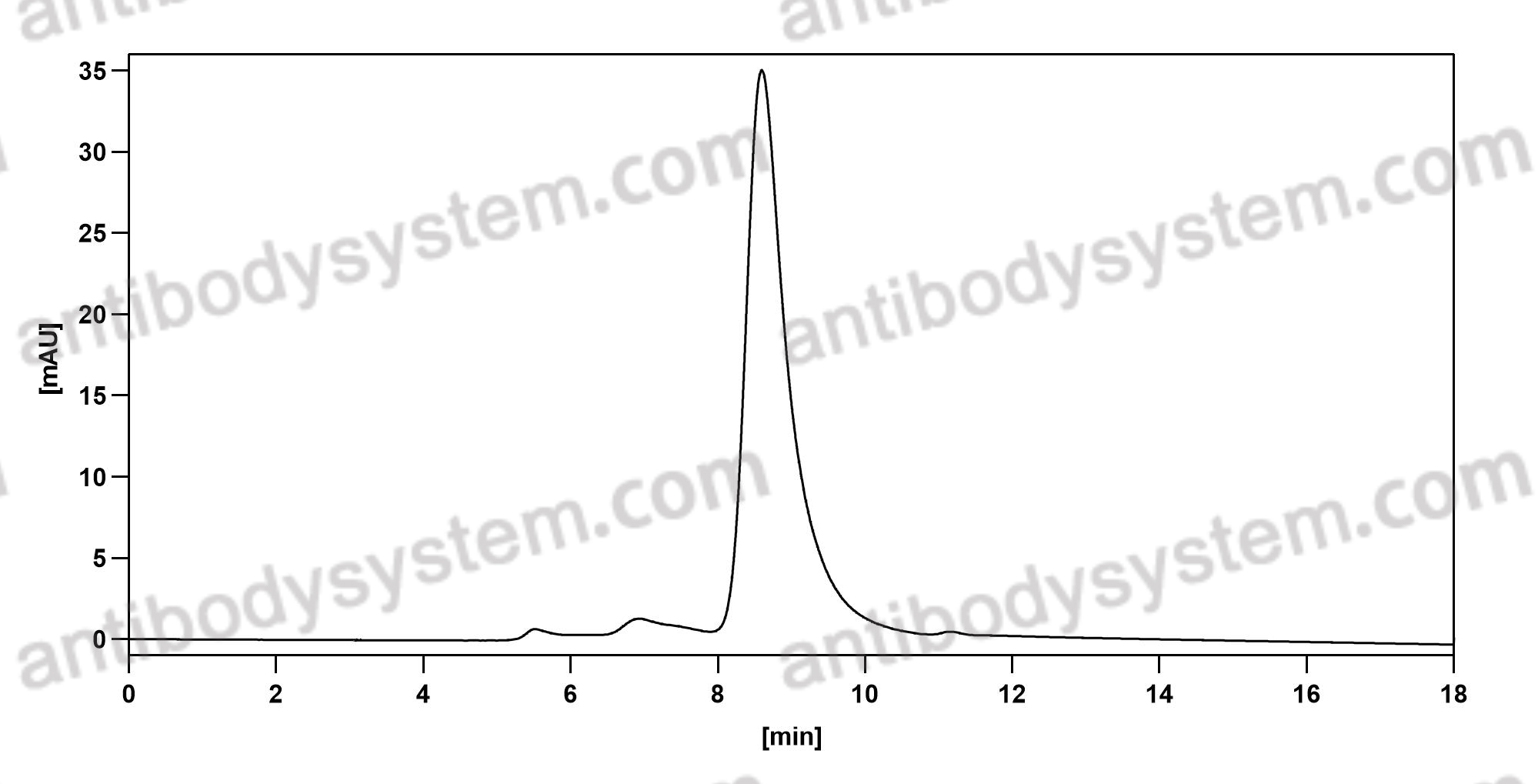
[Update Osteoarthritis], PMID: 32167252
A multiple-dose toxicity study of tanezumab in cynomolgus monkeys, PMID: 21130822
Antibodies to watch in 2019, PMID: 30516432
Antibodies to watch in 2020, PMID: 31847708
Antibodies to watch in 2021, PMID: 33459118
Bioanalytical assays in support of tanezumab developmental and reproductive toxicity studies: challenges and learnings, PMID: 31204868
Clinical Outcomes of Tanezumab With Different Dosages for Patient With Osteoarthritis: Network Meta-Analysis, PMID: 34177562
Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients With Osteoarthritis of the Hip or Knee: A Randomized Clinical Trial, PMID: 31265100
Effects of tanezumab on satellite glial cells in the cervicothoracic ganglion of cynomolgus monkeys: A 26-week toxicity study followed by an 8-week recovery period, PMID: 30890348
Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen, PMID: 25274899
Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial, PMID: 23852695
Efficacy and safety of tanezumab administered as a fixed dosing regimen in patients with knee or hip osteoarthritis: a meta-analysis of randomized controlled phase III trials, PMID: 33159281
Efficacy and safety of tanezumab in the treatment of chronic low back pain, PMID: 21696889
Efficacy and safety of tanezumab in the treatment of pain from bone metastases, PMID: 25919474
Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain, PMID: 24625625
Efficacy and Safety of Tanezumab on Osteoarthritis Knee and Hip Pains: A Meta-Analysis of Randomized Controlled Trials, PMID: 28034979
Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain, PMID: 23628600
Erratum: Pooled Analysis of Tanezumab Efficacy and Safety with Subgroup Analyses of Phase III Clinical Trials in Patients with Osteoarthritis Pain of the Knee or Hip [Corrigendum], PMID: 32982389
Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain, PMID: 25594611
First-in-human randomized clinical trials of the safety and efficacy of tanezumab for treatment of chronic knee osteoarthritis pain or acute bunionectomy pain, PMID: 29922745
From the Cover: Evaluation of the Effects of Tanezumab, a Monoclonal Antibody Against Nerve Growth Factor, on the Sympathetic Nervous System in Adult Cynomolgus Monkeys (Macaca fascicularis): A Stereologic, Histomorphologic, and Cardiofunctional Assessment, PMID: 28525647
Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain, PMID: 24937440
Long-Term Safety and Efficacy of Subcutaneous Tanezumab Versus Nonsteroidal Antiinflammatory Drugs for Hip or Knee Osteoarthritis: A Randomized Trial, PMID: 33538113
Nerve safety of tanezumab, a nerve growth factor inhibitor for pain treatment, PMID: 25073573
NGF/TrkA Signaling as a Therapeutic Target for Pain, PMID: 26452158
Onset and maintenance of efficacy of subcutaneous tanezumab in patients with moderate to severe osteoarthritis of the knee or hip: A 16-week dose-titration study, PMID: 32252976
Pharmacology of nerve growth factor and discovery of tanezumab, an anti-nerve growth factor antibody and pain therapeutic, PMID: 31026504
Pooled analysis of tanezumab efficacy and safety with subgroup analyses of phase III clinical trials in patients with osteoarthritis pain of the knee or hip, PMID: 30936738
Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis, PMID: 21420111
Relative efficacy and tolerability of 2.5, 5, and 10 mg tanezumab for the treatment of osteoarthritis: A Bayesian network meta-analysis of randomized controlled trials based on patient withdrawal, PMID: 33141017
Response to: "Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis", PMID: 30774242
Response to: 'Use of tanezumab for patients with hip and knee osteoarthritis with reference to a randomised clinical trial by Berenbaum and colleagues' by Riddle and Perera, PMID: 32424029
Safety and efficacy of subcutaneous tanezumab in patients with knee or hip osteoarthritis, PMID: 29386912
Safety of Low-Dose Tanezumab in the Treatment of Hip or Knee Osteoarthritis: A Systemic Review and Meta-analysis of Randomized Phase III Clinical Trials, PMID: 33141224
Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period, PMID: 32234715
Subcutaneous tanezumab for osteoarthritis: Is the early improvement in pain and function meaningful and sustained?, PMID: 33728717
Tanezumab for chronic low back pain: a randomized, double-blind, placebo- and active-controlled, phase 3 study of efficacy and safety, PMID: 32453139
Tanezumab for Painful Osteoarthritis, PMID: 31265081
Tanezumab for Patients with Osteoarthritis of the Knee: A Meta-Analysis, PMID: 27294371
Tanezumab for the treatment of pain from osteoarthritis of the knee, PMID: 20942668
Tanezumab improves difficult-to-treat OA, PMID: 32367021
Tanezumab in the treatment of chronic musculoskeletal conditions, PMID: 27936977
Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial, PMID: 23553790
Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial, PMID: 22784777
Tanezumab Reduces Pain in Women with Interstitial Cystitis/Bladder Pain Syndrome and Patients with Nonurological Associated Somatic Syndromes, PMID: 26576710
Tanezumab, a recombinant humanized mAb against nerve growth factor for the treatment of acute and chronic pain, PMID: 20140821
Tanezumab: a selective humanized mAb for chronic lower back pain, PMID: 29503555
Tanezumab: Finally a Monoclonal Antibody for Pain Relief, PMID: 30111960
Tanezumab: Therapy targeting nerve growth factor in pain pathogenesis, PMID: 29643634
Use of tanezumab for patients with hip and knee osteoarthritis with reference to a randomised clinical trial by Berenbaum and colleagues, PMID: 32321719
Associations between testosterone and knee and hand osteoarthritis among males and females from the general population., PMID:40221126
Metabolic syndrome, radiographic osteoarthritis progression and chronic pain of the knee among men and women from the general population: The Rotterdam study., PMID:39288696
Re: Re: Laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs., PMID:38885831
The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain., PMID:38752946
Effect size varies based on calculation method and may affect interpretation of treatment effect: an illustration using randomised clinical trials in osteoarthritis., PMID:38650049
Various Doses of Tanezumab in the Management of Chronic Low Back Pain (CLBP): A Pooled Analysis of 4,514 Patients., PMID:37954824
Characterization of adverse joint outcomes in patients with osteoarthritis treated with subcutaneous tanezumab., PMID:37652258
Prediction of relative change in free nerve growth factor following subcutaneous administration of tanezumab, a novel monoclonal antibody to nerve growth factor., PMID:37470295
Peripheral Nerve Safety of Nerve Growth Factor Inhibition by Tanezumab: Pooled Analyses of Phase III Clinical Studies in Over 5000 Patients with Osteoarthritis., PMID:37460782
A Randomized Placebo-Controlled Trial of the Anti-Nerve Growth Factor Antibody Tanezumab in Subjects With Cancer Pain Due to Bone Metastasis., PMID:37343145
Role of imaging for eligibility and safety of a-NGF clinical trials., PMID:37284331
Examining the Relationships Among Treatment, Pain, and Physical Function in Patients With Osteoarthritis: A Mediation-Modeling Approach., PMID:36806283
The Current Role of Disease-modifying Osteoarthritis Drugs., PMID:36793668
Predicting Treatment Responses in Patients With Osteoarthritis: Results From Two Phase III Tanezumab Randomized Clinical Trials., PMID:36621827
[Clinical characteristics of immune checkpoint inhibitor-related type 1 diabetes mellitus]., PMID:36594136
Different Dosage Regimens of Tanezumab for the Treatment of Chronic Low Back Pain: A Meta-analysis of Randomized Controlled Trials., PMID:36542785
Time to first and sustained improvement in WOMAC domains among patients with osteoarthritis receiving tanezumab., PMID:36474952
A Two-Step, Trajectory-Focused, Analytics Approach to Attempt Prediction of Analgesic Response in Patients with Moderate-to-Severe Osteoarthritis., PMID:36301512
Nerve Growth Factor (NGF) Encourages the Neuroinvasive Potential of Pancreatic Cancer Cells by Activating the Warburg Effect and Promoting Tumor Derived Exosomal miRNA-21 Expression., PMID:36285300
Inter-Reader Consistency and Exclusionary Findings During Radiographic Screening for Phase 3 Trials of Tanezumab in Patients With Osteoarthritis., PMID:38343426
Design of a randomized, placebo-controlled, phase 2 study evaluating the safety and efficacy of tanezumab for treatment of schwannomatosis-related pain., PMID:36038003
Efficacy and safety of tanezumab, NSAIDs, and placebo in patients with moderate to severe hip or knee osteoarthritis and a history of depression, anxiety, or insomnia: post-hoc analysis of phase 3 trials., PMID:35980115
Clinical Meaningfulness of Response to Tanezumab in Patients with Chronic Low Back Pain: Analysis From a 56-Week, Randomized, Placebo- and Tramadol-Controlled, Phase 3 Trial., PMID:35962939
Characterizing 16-Week Responder Profiles Using Group-Based Trajectory Modeling in Over 4300 Clinical Trial Participants Receiving Pharmaceutical Treatment for Moderate to Severe Osteoarthritis., PMID:35960482
Trends of Dispensed Opioids in Catalonia, Spain, 2007-19: A Population-Based Cohort Study of Over 5 Million Individuals., PMID:35754470
Monoclonal Antibody Therapy for the Treatment of Interstitial Cystitis., PMID:35619987
Efficacy, General Safety, and Joint Safety of Tanezumab in Japanese Patients with Osteoarthritis: Subgroup Analyses from Two Randomized, Phase 3 Studies., PMID:35538185
Efficacy and Safety of Anti-Nerve Growth Factor Antibody Therapy for Hip and Knee Osteoarthritis: A Meta-analysis., PMID:35494494
Gout and Hospital Admission for Ambulatory Care-Sensitive Conditions: Risks and Trajectories., PMID:35428711
Analgesic effects of nerve growth factor-directed monoclonal antibody on diabetic neuralgia in an animal model., PMID:35417079
Tanezumab for the treatment of osteoarthritis pain., PMID:35412532
Comorbidities in osteoarthritis (ComOA): a combined cross-sectional, case-control and cohort study using large electronic health records in four European countries., PMID:35387809
Observed efficacy and clinically important improvements in participants with osteoarthritis treated with subcutaneous tanezumab: results from a 56-week randomized NSAID-controlled study., PMID:35351194
Peripheral Voltage-Gated Cation Channels in Neuropathic Pain and Their Potential as Therapeutic Targets., PMID:35295464
[Osteoarthritis: what's new?]., PMID:35291041
WOMAC Meaningful Within-patient Change: Results From 3 Studies of Tanezumab in Patients With Moderate-to-severe Osteoarthritis of the Hip or Knee., PMID:35232805
Neurological safety of subcutaneous tanezumab versus NSAID in patients with osteoarthritis., PMID:35217440
Development of radiographic classification criteria for hand osteoarthritis: a methodological report (Phase 2)., PMID:35121640
Population pharmacokinetics of tanezumab following intravenous or subcutaneous administration to patients with osteoarthritis or chronic low back pain., PMID:35112378
Impact of tanezumab on health status, non-work activities and work productivity in adults with moderate-to-severe osteoarthritis., PMID:35105318
Based on minimal clinically important difference values, a moderate dose of tanezumab may be a better option for treating hip or knee osteoarthritis: a meta-analysis of randomized controlled trials., PMID:35069811
Effectiveness of Various Dosages and Administration Methods of Tanezumab for the Treatment of Pain in Knee and Hip Osteoarthritis: a Network Meta-Analysis., PMID:34819241
Nerve Growth Factor (NGF) Inhibitors and Related Agents for Chronic Musculoskeletal Pain: A Comprehensive Review., PMID:34807432
Tanezumab for chronic low back pain: a long-term, randomized, celecoxib-controlled Japanese Phase III safety study., PMID:34786956
Different Drugs for the Treatment of Painful Diabetic Peripheral Neuropathy: A Meta-Analysis., PMID:34777192
Association of Tramadol vs Codeine Prescription Dispensation With Mortality and Other Adverse Clinical Outcomes., PMID:34665205
Preparation and characterization of a high-affinity monoclonal antibody against nerve growth factor., PMID:34627999
Gender, age, disease severity, body mass index and diabetes may not affect response to subcutaneous tanezumab in patients with osteoarthritis after 16 weeks of treatment. A subgroup analysis of placebo-controlled trials., PMID:34626502
Relative Efficacy and Safety of Tanezumab for Osteoarthritis: A Systematic Review and Meta-analysis of Randomized-Controlled Trials., PMID:34608021
Single and Composite Endpoints of Within-Patient Improvement in Symptoms: Pooled Tanezumab Data in Patients with Osteoarthritis., PMID:34606077