Catalog No.
DHD68911
Description
The FDA has granted an orphan drug designation to sotigalimab (APX005M) as a potential therapeutic option for patients with soft tissue sarcoma, according to announcement from Apexigen Inc., the drug developer.
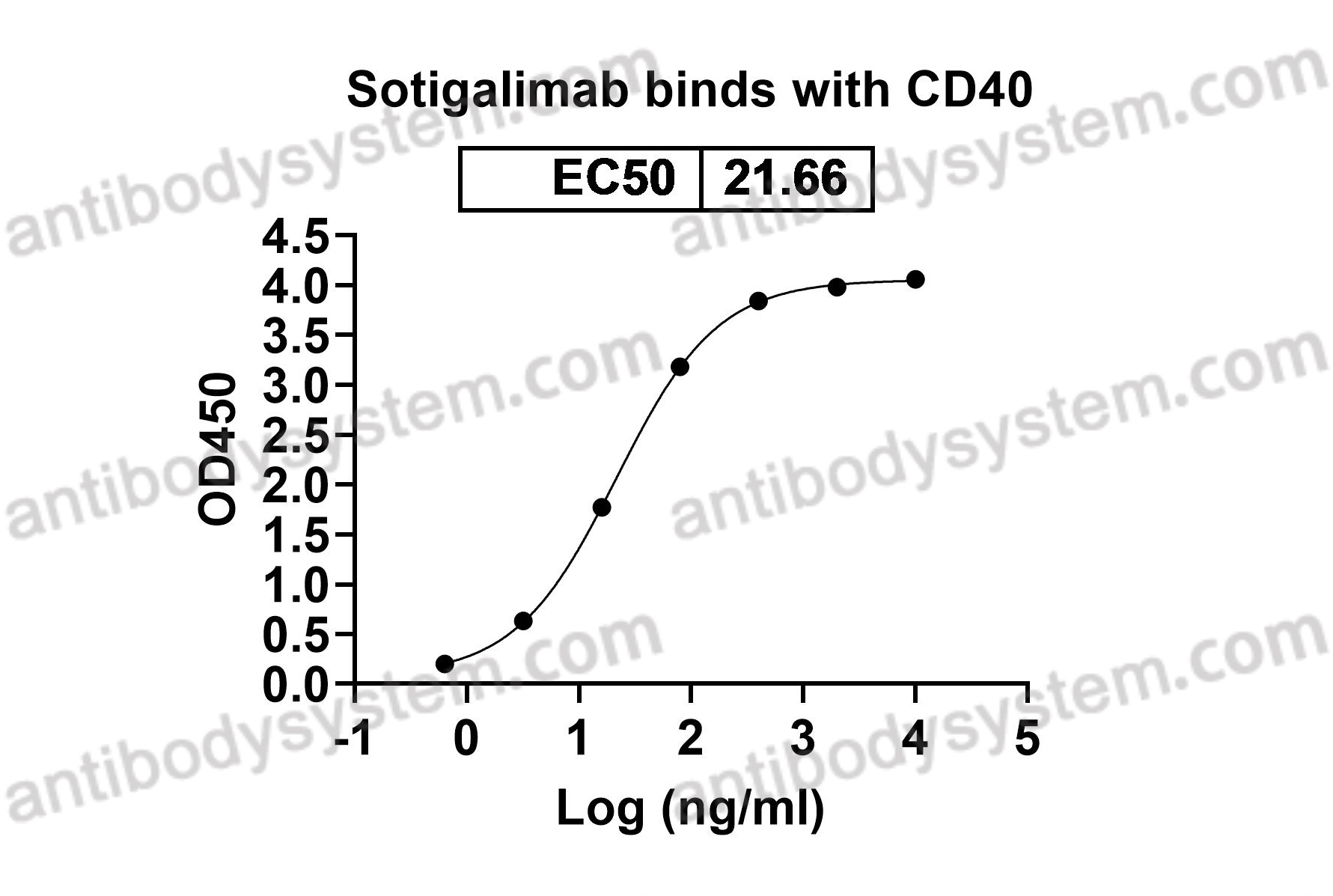
A novel, humanized monoclonal antibody, sotigalimab was designed stimulate antitumor immune response and to target CD40, which is a key co-stimulatory receptor that serves to activate innate and adaptive immune systems. When sotigalimab binds to CD40 on antigen-presenting cells, it induces a multifaceted immune response that brings several components of the immune system together to attack the cancer.
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-Kappa
Clonality
Monoclonal
Target
Tumor necrosis factor receptor superfamily member 5, B-cell surface antigen CD40, Bp50, CD40L receptor, TNFRSF5, CDw40, CD40
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
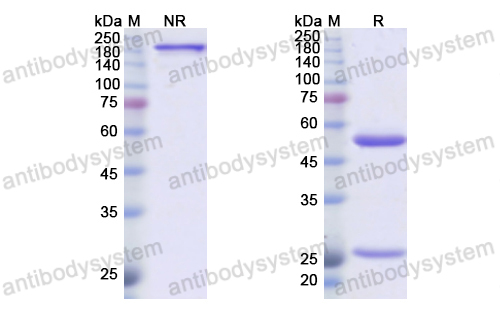
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P25942
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
APX005M, APX-005, APX-005-M, APX-005M, CAS: 2305607-45-6
Clone ID
Sotigalimab
CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study, PMID: 33387490
A CD40 Agonistic Antibody Shows Signs of Efficacy in Pancreatic Cancer, PMID: 33451984
A Phase I Study of APX005M and Cabiralizumab with or without Nivolumab in Patients with Melanoma, Kidney Cancer, or Non-Small Cell Lung Cancer Resistant to Anti-PD-1/PD-L1, PMID: 34140403
A Phase 2 Study of Sotigalimab, a CD40 Agonist Antibody, plus Concurrent Chemoradiation as Neoadjuvant Therapy for Esophageal and Gastroesophageal Junction Cancers., PMID:39907035
Neoadjuvant CD40 Agonism Remodels the Tumor Immune Microenvironment in Locally Advanced Esophageal/Gastroesophageal Junction Cancer., PMID:38181044
A bedside to bench study of anti-PD-1, anti-CD40, and anti-CSF1R indicates that more is not necessarily better., PMID:37964379
A Phase II Trial of the CD40 Agonistic Antibody Sotigalimab (APX005M) in Combination with Nivolumab in Subjects with Metastatic Melanoma with Confirmed Disease Progression on Anti-PD-1 Therapy., PMID:37535056
Two types of biomarker-dependent chemo-immunotherapy for pancreatic cancer?, PMID:36260984
Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial., PMID:35662283
A Phase I Study of APX005M and Cabiralizumab with or without Nivolumab in Patients with Melanoma, Kidney Cancer, or Non-Small Cell Lung Cancer Resistant to Anti-PD-1/PD-L1., PMID:34140403
CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study., PMID:33387490