Catalog No.
DHB34101
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG2-Kappa
Clonality
Monoclonal
Target
TNF- and APOL-related leukocyte expressed ligand 2, APRIL, CD256, Tumor necrosis factor ligand superfamily member 13, TNF-related death ligand 1, TALL-2, TNFSF13, ZTNF2, A proliferation-inducing ligand, TALL2, TRDL-1
Concentration
1.5 mg/ml
Endotoxin level
Please contact with the lab for this information.
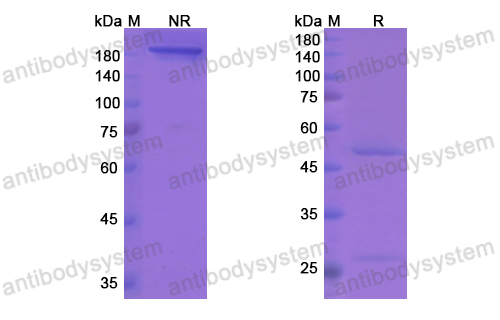
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
O75888
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
VIS-649, CAS: 2382896-07-1
Clone ID
Sibeprenlimab
Safety Reporting for Auxiliary Medicinal Products in the Sibeprenlimab Phase-III Study According to the New European Union (536/2014) CT Regulation., PMID:40304336
[The highlights of nephrology in 2024]., PMID:40208671
A novel agent targeting APRIL: A new hope for elderly patients of IgA nephropathy., PMID:39949470
[Nephrology : what's new in 2024 (II)]., PMID:39846098
Biologics and Non-Biologics Immunosuppressive Treatments for IgA Nephropathy in Both Adults and Children., PMID:38730994
Sibeprenlimab, which neutralizes A PRoliferation Inducing Ligand (APRIL), as a new approach to treating IgA nephropathy., PMID:38641998
Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy. Reply., PMID:38598588
Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy., PMID:38598587
[Immunoglobulin A nephropathy-New treatment possibilities]., PMID:38294502
The contribution of a proliferation-inducing ligand (APRIL) and other TNF superfamily members in pathogenesis and progression of IgA nephropathy., PMID:38053976
A Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy., PMID:37916620
Safety, Pharmacokinetics, and Pharmacodynamics of Subcutaneous Sibeprenlimab in Healthy Participants., PMID:37565623
Emerging role of monoclonal antibodies in the treatment of IgA nephropathy., PMID:37183663
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VIS649 (Sibeprenlimab), an APRIL-Neutralizing IgG2 Monoclonal Antibody, in Healthy Volunteers., PMID:35570983