Catalog No.
DHC13603
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-Kappa
Clonality
Monoclonal
Target
IL5, B-cell differentiation factor I, IL-5, T-cell replacing factor, TRF, Eosinophil differentiation factor, Interleukin-5
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
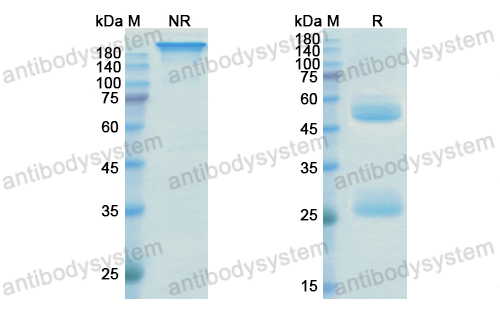
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P05113
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
GSK-3511294, CAS: 2243274-14-6
Clone ID
Depemokimab
Mechanisms and Treatment of Type 2 High and Low Asthma Endotypes., PMID:40462371
Biologic Therapies for Severe Asthma: Current Insights and Future Directions., PMID:40364184
Depemokimab in chronic rhinosinusitis with nasal polyps: A turning point or a missed opportunity?, PMID:40347936
Reappraisal of Biologic Efficacy from Phase 3 Trials in Refractory Chronic Rhinosinusitis and Nasal Polyps., PMID:40340024
Depemokimab in eosinophilic asthma - a new era in biological therapy?, PMID:40260270
Efficacy and safety of twice per year depemokimab in chronic rhinosinusitis with nasal polyps (ANCHOR-1 and ANCHOR-2): phase 3, randomised, double-blind, parallel trials., PMID:40037388
Pharmacokinetics of Depemokimab Delivered by Safety Syringe Device or Autoinjector in Healthy Adults: A Phase 1, Single-Dose Study., PMID:39876532
In severe asthma with an eosinophilic phenotype, depemokimab improved exacerbations, but not quality of life, at 52 wk., PMID:39761576
Depemokimab, the first ultra-long-acting anti-IL-5 monoclonal antibody for severe eosinophilic asthma., PMID:39674169
Drugs for asthma., PMID:39558440
Twice-Yearly Depemokimab in Severe Asthma with an Eosinophilic Phenotype., PMID:39248309
World Health Organization and International Consensus Classification of eosinophilic disorders: 2024 update on diagnosis, risk stratification, and management., PMID:38551368