Catalog No.
DHD58802
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-Kappa
Clonality
Monoclonal
Target
CD252, OX40L, TXGP1, OX40 ligand, Glycoprotein Gp34, TAX transcriptionally-activated glycoprotein 1, TNFSF4, Tumor necrosis factor ligand superfamily member 4
Concentration
2.9 mg/ml
Endotoxin level
Please contact with the lab for this information.
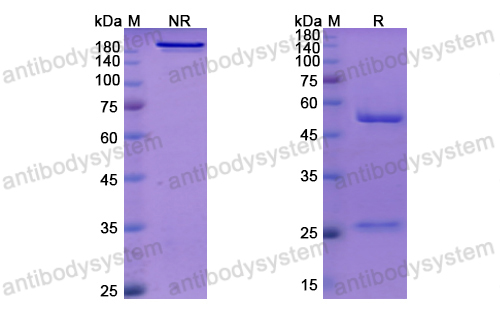
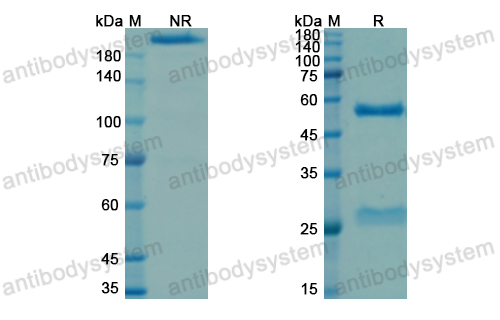
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P23510
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
KY-1005, KY1005, KY1005-2D10,KY-1005-2D10, CAS: 2378692-15-8
Clone ID
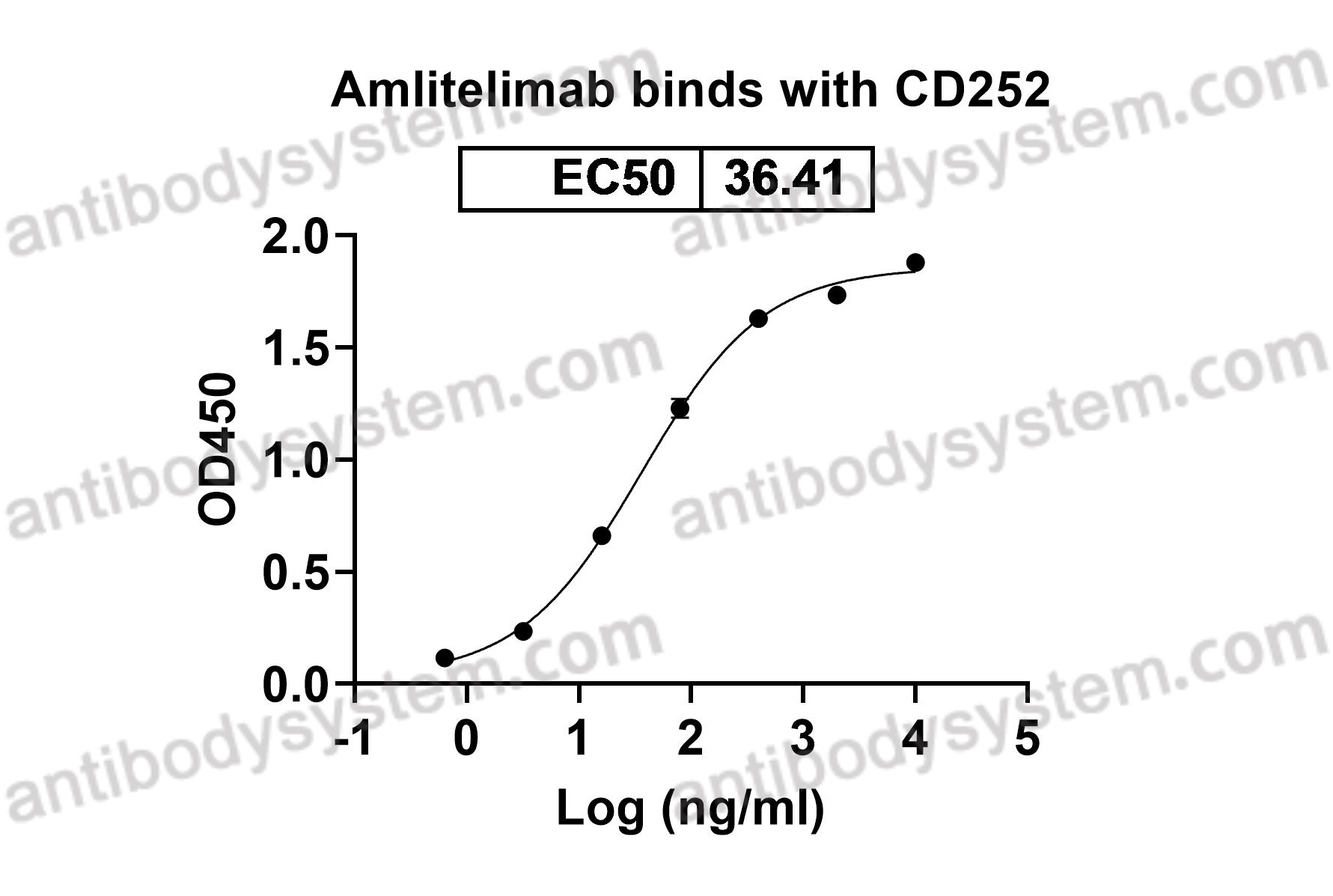
Amlitelimab
OX40/OX40L as a Therapeutic Target in Atopic Dermatitis: A Scoping Review., PMID:40331215
Safety and Efficacy of Anti-OX40 Therapies in Atopic Dermatitis: A Systematic Review and Meta-Analysis., PMID:40229131
Investigating Efficacy of Atopic Dermatitis Systemic Therapeutics After Discontinuation Part I: Biologics., PMID:40078859
Targeting the OX40-OX40L pathway: A new era in atopic dermatitis management by T-cell rebalancing., PMID:39978683
A Narrative Review of the OX40-OX40L Pathway as a Potential Therapeutic Target in Atopic Dermatitis: Focus on Rocatinlimab and Amlitelimab., PMID:39565527
Design of CONQUEST, a novel, randomized, placebo-controlled, Phase 2b platform clinical trial to investigate new treatments for patients with early active systemic sclerosis with interstitial lung disease., PMID:39544897
Phase 2b randomized clinical trial of amlitelimab, an anti-OX40 ligand antibody, in patients with moderate-to-severe atopic dermatitis., PMID:39522654
A Comprehensive Review of Biologics in Phase III and IV Clinical Trials for Atopic Dermatitis., PMID:39064040
Emerging drugs for the treatment of atopic dermatitis: a focus on phase 2 and phase 3 trials., PMID:38662529
OX40 in the Pathogenesis of Atopic Dermatitis-A New Therapeutic Target., PMID:38236520
An overview of new and emerging antibody therapies for moderate-severe atopic dermatitis in adults., PMID:38054328
Safety and effectiveness of amlitelimab, a potential new treatment for atopic dermatitis: results of a clinical trial., PMID:37879745
Safety and efficacy of amlitelimab, a fully human nondepleting, noncytotoxic anti-OX40 ligand monoclonal antibody, in atopic dermatitis: results of a phase IIa randomized placebo-controlled trial., PMID:37463508
OX40-OX40L Inhibition for the Treatment of Atopic Dermatitis-Focus on Rocatinlimab and Amlitelimab., PMID:36559247
OX40L Inhibition Suppresses KLH-driven Immune Responses in Healthy Volunteers: A Randomized Controlled Trial Demonstrating Proof-of-Pharmacology for KY1005., PMID:35092305