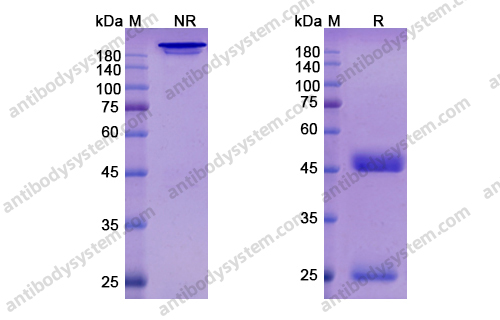
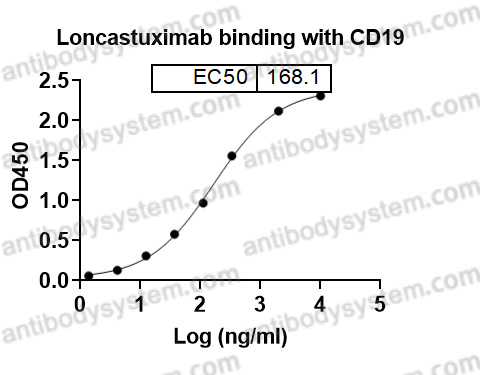
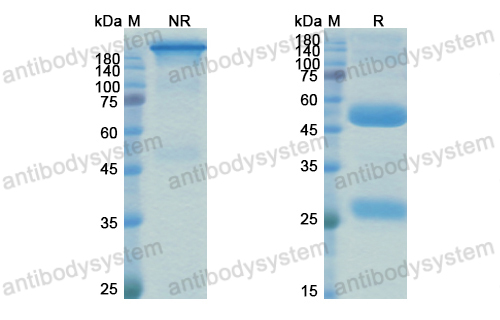
Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma, PMID: 33211842
Loncastuximab tesirine, an anti-CD19 antibody-drug conjugate, in relapsed/refractory B-cell acute lymphoblastic leukemia, PMID: 32012214
A Phase I Study of ADCT-402 (Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody-Drug Conjugate, in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma, PMID: 31685491
Loncastuximab tesirine in patients with B-cell non-Hodgkin lymphoma, PMID: 30543588
Antibodies to watch in 2020, PMID: 31847708
Loncastuximab Tesirine: First Approval, PMID: 34143407
Loncastuximab Tesirine-lpyl, PMID: 34131694
Loncastuximab Tesirine, PMID: 34014627
ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies, PMID: 29298756
Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial, PMID: 33989558
FDA approves ADC Therapeutics' loncastuximab tesirine, ushering in a new cytotoxic payload, PMID: 33981088
Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy, PMID: 32543981
The Role of Specific ATP-Binding Cassette Transporters in the Acquired Resistance to Pyrrolobenzodiazepine Dimer-Containing Antibody-Drug Conjugates, PMID: 32669316
Diffuse Telangiectatic Rash Associated With Novel Antibody Drug Conjugate Therapies, PMID: 32267477
Chemotherapy-Free Management of Follicular and Marginal Zone Lymphoma, PMID: 34017197
Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress, PMID: 34090506
The era of CD19-directed therapy in diffuse large B-cell lymphoma, PMID: 33989556
QSP modeling of loncastuximab tesirine with T-cell-dependent bispecific antibodies guides dose-regimen strategy., PMID:40500294
Antibody-Directed Cell Internalization of Targeted Covalent Europium Tag Enables In Situ Kinase Labeling and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Quantification., PMID:40470552
Real-world treatment of large B-cell lymphoma with chimeric antigen receptor T-cell therapy after loncastuximab tesirine: a plain language summary., PMID:40444351
How well does loncastuximab tesirine work after chimeric antigen receptor T-cell treatment in diffuse large B-cell lymphoma? A plain language summary of a real-world evidence study., PMID:40439268
Management of Diffuse Large B-Cell Lymphoma as Post-Transplant Lymphoproliferative Disorder in a Kidney Transplant Recipient: A Case Report., PMID:40407632
Response to Michelerio et al regarding "Antibody-drug conjugates: A review of cutaneous adverse effects"., PMID:40239815
Response to Saberi et al, "Antibody-drug conjugates: A review of cutaneous adverse effects"., PMID:40233839
Budget impact of introducing glofitamab for treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy in the United States., PMID:40163049
Loncastuximab Tesirine Versus Polatuzumab Vedotin Plus Bendamustine and Rituximab in Relapsed/Refractory DLBCL After ≥ 2 Lines of Therapy: Matching-Adjusted Indirect Comparison., PMID:40153222
Global, regional, and national prevalence of adult overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021., PMID:40049186
Global, regional, and national prevalence of child and adolescent overweight and obesity, 1990-2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021., PMID:40049185
Global, regional, and national burden of epilepsy, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021., PMID:40015291
Changing life expectancy in European countries 1990-2021: a subanalysis of causes and risk factors from the Global Burden of Disease Study 2021., PMID:39983748
Real-World Effectiveness of Chemoimmunotherapy and Novel Therapies for Patients With Relapsed/Refractory Aggressive Large B-Cell Lymphoma., PMID:39966020
The potential of antibody-drug conjugates for effective therapy in diffuse large B-cell lymphoma., PMID:39798075
Synthesis of novel pyrrolobenzodiazepine (PBD) C1-substituted monomers and dimers with DNA-binding activity and cytotoxicity., PMID:39778751
Loncastuximab tesirine with rituximab in patients with relapsed or refractory follicular lymphoma: a single-centre, single-arm, phase 2 trial., PMID:39662486
SOHO State of the Art Updates and Next Questions | Diffuse Large B-Cell Lymphoma in Older Adults: A Comprehensive Review., PMID:39613700
Outcomes with loncastuximab tesirine following CAR T-cell therapy in patients with relapsed or refractory diffuse large B-cell lymphoma., PMID:39609431
Loncastuximab in high-risk and heavily pretreated relapsed/refractory diffuse large B-cell lymphoma: a realworld analysis from 21 US centers., PMID:39540227
Loncastuximab tesirine in previously treated diffuse large B-cell lymphoma: A plain language summary of the LOTIS-2 study., PMID:39530615
Understanding how CD19 expression levels impact the response to loncastuximab tesirine: a plain language summary., PMID:39508421
Treatment of Secondary Hemophagocytic Lymphohistiocytosis Associated With Diffuse Large B-cell Lymphoma Using Loncastuximab Tesirine As Lymphoma-Directed Therapy., PMID:39473653
Vesiculobullous eruption with loncastuximab tesirine in a patient with relapsed follicular lymphoma., PMID:39421259
Real-world treatment of large B-cell lymphoma with chimeric antigen receptor T-cell therapy after loncastuximab tesirine., PMID:39415934
Forecasting the effects of smoking prevalence scenarios on years of life lost and life expectancy from 2022 to 2050: a systematic analysis for the Global Burden of Disease Study 2021., PMID:39366729
How I treat older patients with relapsed/refractory diffuse large B-cell lymphoma., PMID:39356892
Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021., PMID:39304265
Loncastuximab tesirine in Chinese patients with relapsed or refractory diffuse large B-cell lymphoma: a multicenter, open-label, single-arm, phase II trial., PMID:39234866
Cutaneous Collagenous Vasculopathy Associated With Antibody-Drug Conjugate Treatment., PMID:39167367
Loncastuximab Tesirine in the Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma., PMID:39062823
Allogeneic transplantation after immunotherapy for relapsed/refractory non-Hodgkin lymphoma: a comparison with a historical cohort., PMID:38775776
Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021., PMID:38762324
Targeting CD19-positive lymphomas with the antibodydrug conjugate loncastuximab tesirine: preclinical evidence of activity as a single agent and in combination therapy., PMID:38721745
Sequencing of Anti-CD19 Therapies in the Management of Diffuse Large B-Cell Lymphoma., PMID:38661647
Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021., PMID:38642570
Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021., PMID:38582094
Global fertility in 204 countries and territories, 1950-2021, with forecasts to 2100: a comprehensive demographic analysis for the Global Burden of Disease Study 2021., PMID:38521087
In relapsed or refractory diffuse large B-cell lymphoma, CD19 expression by immunohistochemistry alone is not a predictor of response to loncastuximab tesirine., PMID:38406517
Second-line treatment of diffuse large B-cell lymphoma: Evolution of options., PMID:38342663
Loncastuximab tesirine: Risk for dose variance., PMID:38298157
Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: a real-world pharmacovigilance evaluation of the FDA adverse event reporting system., PMID:38100054
Efficacy of CD19 directed therapies in patients with relapsed or refractory large b-cell lymphoma relapsing after CD19 directed chimeric antigen receptor T-cell therapy., PMID:37973893
What's new in the use of antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) DNA cross-linkers for cancer therapy?, PMID:37902460
Clinical outcomes of older and younger patients treated with loncastuximab tesirine in the LOTIS-2 clinical trial., PMID:37871303
PET/CT Biomarkers Enable Risk Stratification of Patients with Relapsed/Refractory Diffuse Large B-cell Lymphoma Enrolled in the LOTIS-2 Clinical Trial., PMID:37855688
Outcome after chimeric antigen receptor (CAR) T-cell therapy failure in large B-cell lymphomas., PMID:37690811
Loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma: long-term efficacy and safety from the phase II LOTIS-2 study., PMID:37646659