Catalog No.
DGK07803
Expression system
Mammalian Cells
Species reactivity
General
Host species
Chimeric
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Ganglioside GD2
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
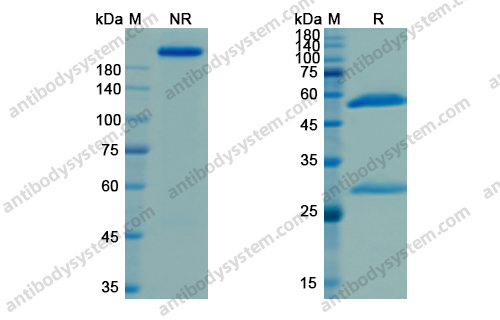
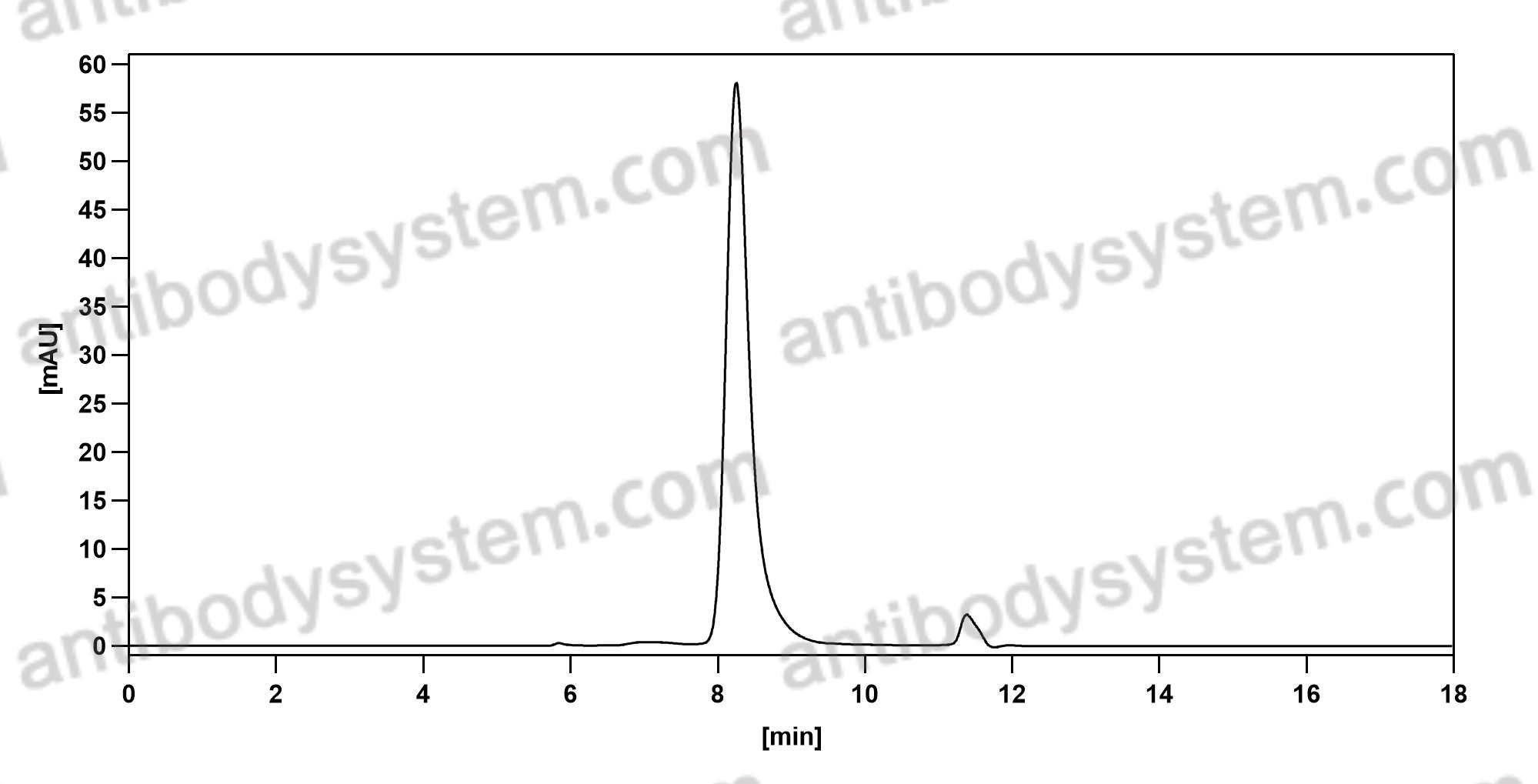
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
CAS: 65988-71-8
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Hu3F8, Naxitamab-gqgk, CAS: 1879925-92-4
Clone ID
Naxitamab
Naxitamab, PMID: 33355727
Naxitamab-gqgk, PMID: 33684934
Naxitamab: First Approval, PMID: 33616889
Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression, PMID: 33326254
Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission, PMID: 34022112
The Role of Autologous Stem-Cell Transplantation in High-Risk Neuroblastoma Consolidated by anti-GD2 Immunotherapy. Results of Two Consecutive Studies, PMID: 33324208
Clinical and Pathological Evidence of Anti-GD2 Immunotherapy Induced Differentiation in Relapsed/Refractory High-Risk Neuroblastoma, PMID: 33809255
A Phase II Trial of Naxitamab plus Stepped-up Dosing of GM-CSF for Patients with High-Risk Neuroblastoma in First Complete Remission., PMID:40067131
The anti-GD2 monoclonal antibody naxitamab plus GM-CSF for relapsed or refractory high-risk neuroblastoma: a phase 2 clinical trial., PMID:39952926
Case Report: Successful treatment of metastatic retinoblastoma with CNS involvement with anti-GD2 immunotherapy, intrathecal topotecan and reduced systemic chemotherapy., PMID:39895987
Adverse Reaction Reporting for Naxitamab in Chinese Expanded Access Treatment for Relapsed/Refractory High-Risk Neuroblastoma at the Children's Hospital of Fudan University., PMID:39704915
GD2 in Breast Cancer: A Potential Biomarker and Therapeutic Target., PMID:39467630
Omalizumab for Treatment of Anti-GD2 Antibody-related Urticaria., PMID:39177945
Desensitizing the autonomic nervous system to mitigate anti-GD2 monoclonal antibody side effects., PMID:38812778
Guidelines for outpatient administration of naxitamab: Experience from Atrium Health Levine Children's Hospital., PMID:38396377
An Expedition on Synthetic Methodology of FDA-approved Anticancer Drugs (2018-2021)., PMID:38288815
Partial Response to Naxitamab for Brain Metastasis in Neuroblastoma., PMID:38189408
Toxicity Spectrum of Anti-GD2 Immunotherapy: A Real-World Study Leveraging the US Food and Drug Administration Adverse Event Reporting System., PMID:38153627
GM-CSF, G-CSF or no cytokine therapy with anti-GD2 immunotherapy for high-risk neuroblastoma., PMID:38108214
Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes., PMID:37835531
Novel infusion strategy reduces severe adverse events caused by the anti-GD2 monoclonal antibody naxitamab., PMID:37213300
Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use-Updated Outcome Report., PMID:37174002
Correlation between ARID1B gene mutation (p.A460, p.V215G) and prognosis of high-risk refractory neuroblastoma., PMID:36883912
Outcomes of intraventricular 131-I-omburtamab and external beam radiotherapy in patients with recurrent medulloblastoma and ependymoma., PMID:36853490
Naxitamab Activity in Neuroblastoma Cells Is Enhanced by Nanofenretinide and Nanospermidine., PMID:36839972
Pain mitigation and management strategies for anti-GD2 infusions: An expert consensus., PMID:36772891
FDA regulatory considerations for the review of drugs intended to treat pediatric cancers and rare tumors., PMID:36592026
GD2 Expression in Medulloblastoma and Neuroblastoma for Personalized Immunotherapy: A Matter of Subtype., PMID:36551537
Multidisciplinary Clinical Care in the Management of Patients Receiving Anti-GD2 Immunotherapy for High-Risk Neuroblastoma., PMID:36434427
Immunotherapy with anti-GD2 monoclonal antibody in infants with high-risk neuroblastoma., PMID:35913764
Corrigendum to "How we approach the treatment of patients with high-risk neuroblastoma with naxitamab: experience from the Hospital Sant Joan de Déu in Barcelona, Spain": [ESMO Open, 7 (2022) 100462]., PMID:35644100
Outpatient administration of naxitamab in combination with granulocyte-macrophage colony-stimulating factor in patients with refractory and/or relapsed high-risk neuroblastoma: Management of adverse events., PMID:35579862
How we approach the treatment of patients with high-risk neuroblastoma with naxitamab: experience from the Hospital Sant Joan de Déu in Barcelona, Spain., PMID:35397431
Anti-GD2 Directed Immunotherapy for High-Risk and Metastatic Neuroblastoma., PMID:35327550
Naxitamab: a humanized anti-glycolipid disialoganglioside (anti-GD2) monoclonal antibody for treatment of neuroblastoma., PMID:34821881
Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission., PMID:34022112
Potent antitumor effect of T cells armed with anti-GD2 bispecific antibody., PMID:33844437
Clinical and Pathological Evidence of Anti-GD2 Immunotherapy Induced Differentiation in Relapsed/Refractory High-Risk Neuroblastoma., PMID:33809255
Naxitamab-gqgk., PMID:33684934
Naxitamab: First Approval., PMID:33616889
Survival Impact of Anti-GD2 Antibody Response in a Phase II Ganglioside Vaccine Trial Among Patients With High-Risk Neuroblastoma With Prior Disease Progression., PMID:33326254
The Role of Autologous Stem-Cell Transplantation in High-Risk Neuroblastoma Consolidated by anti-GD2 Immunotherapy. Results of Two Consecutive Studies., PMID:33324208