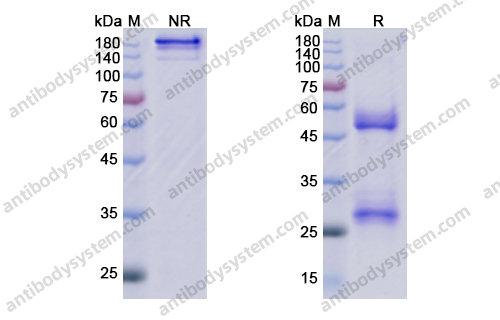
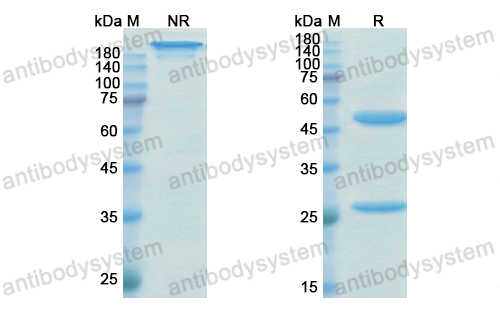
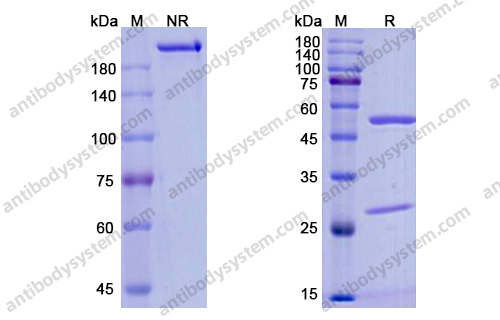
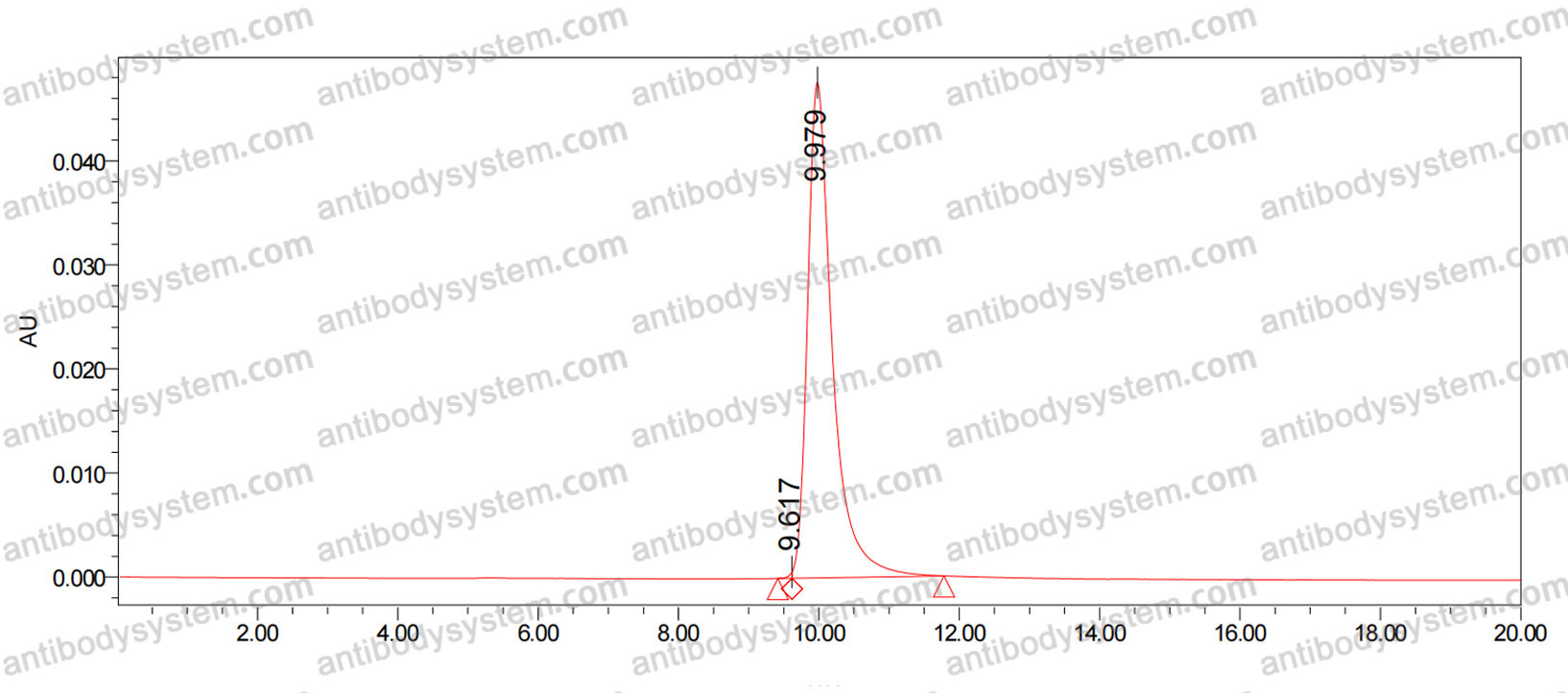
Eptinezumab: First Approval, PMID: 32266704
Eptinezumab for the treatment of migraine, PMID: 31840684
Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2, PMID: 32209650
Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1), PMID: 32075406
Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial, PMID: 31234642
Eptinezumab, PMID: 32697467
Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study, PMID: 33023473
Eptinezumab for the preventive treatment of migraine, PMID: 33280422
Eptinezumab (Vyepti) for migraine prevention, PMID: 32555116
Migraine overview and summary of current and emerging treatment options, PMID: 30681821
Antibodies to watch in 2020, PMID: 31847708
Eptinezumab Demonstrated Efficacy in Sustained Prevention of Episodic and Chronic Migraine Beginning on Day 1 After Dosing, PMID: 33165938
Eptinezumab for the Prevention of Episodic Migraine: Sustained Effect Through 1 Year of Treatment in the PROMISE-1 Study, PMID: 33250209
Eptinezumab, PMID: 34292696
Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine, PMID: 32155317
Monoclonal antibodies for the prevention of migraine, PMID: 31550937
CGRP as the target of new migraine therapies - successful translation from bench to clinic, PMID: 29691490
What's New in the Treatment of Migraine?, PMID: 31393282
Role of CGRP in Migraine, PMID: 30725283
Drugs for Migraine, PMID: 33434187
Antibodies to watch in 2019, PMID: 30516432
Pharmacokinetics, Pharmacodynamics and Drug-Drug Interactions of New Anti-Migraine Drugs-Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies, PMID: 33287305
Eptinezumab for the treatment of migraine, PMID: 34009094
Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans, PMID: 32173558
Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication-overuse headache: Subgroup analysis of PROMISE-2, PMID: 33314079
History and Review of anti-Calcitonin Gene-Related Peptide (CGRP) Therapies: From Translational Research to Treatment, PMID: 30242830
Monoclonal antibodies blocking CGRP transmission: An update on their added value in migraine prevention, PMID: 32758365
European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention, PMID: 30651064
Recent Advances in Pharmacotherapy for Episodic Migraine, PMID: 31556018
Targeting CGRP for the Prevention of Migraine and Cluster Headache: A Narrative Review, PMID: 31291020
Safety and tolerability of eptinezumab in patients with migraine: a pooled analysis of 5 clinical trials, PMID: 33781209
Monoclonal antibodies as a preventive therapy for migraine: A meta-analysis, PMID: 32460120
Effects of Intravenous Eptinezumab vs Placebo on Headache Pain and Most Bothersome Symptom When Initiated During a Migraine Attack: A Randomized Clinical Trial, PMID: 34128999
Immunogenicity of biologic therapies for migraine: a review of current evidence, PMID: 33413094
Tolerability of eptinezumab in overweight, obese or type 1 diabetes patients, PMID: 33855218
Different dosage regimens of Eptinezumab for the treatment of migraine: a meta-analysis from randomized controlled trials, PMID: 33676408
Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine-The monoclonal antibodies and gepants, PMID: 33008505
Calcitonin Gene-Related Peptide Modulators - The History and Renaissance of a New Migraine Drug Class, PMID: 31020659
Patient-identified most bothersome symptom in preventive migraine treatment with eptinezumab: A novel patient-centered outcome, PMID: 34013992
CGRP, a target for preventive therapy in migraine and cluster headache: Systematic review of clinical data, PMID: 29110503
Long-term safety and tolerability of eptinezumab in patients with chronic migraine: a 2-year, open-label, phase 3 trial, PMID: 33740902
Intravenous Migraine Treatment in Children and Adolescents, PMID: 32638172
[Novel migraine treatment with CGRP-related monoclonal antibodies], PMID: 32893246
Rimegepant (Nurtec ODT) for acute treatment of migraine, PMID: 32555113
CGRP, Amylin, Immunology, and Headache Medicine, PMID: 30390312
Antigens and Antibodies in Disease With Specifics About CGRP Immunology, PMID: 30187471
CGRP antagonists for decreasing migraine frequency: New options, long overdue, PMID: 32238376
Monoclonal Antibodies to Prevent Migraine Headaches, PMID: 30855775
Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives, PMID: 32434430
Calcitonin-gene-related peptide pathway mAbs and migraine prevention, PMID: 29432219
Repurposed versus disease-specific medicinals for the prophylaxis of migraine: an updated systematic review., PMID:40447296
Pharmacokinetics and safety of eptinezumab in children and adolescents with migraine., PMID:40444655
Postmarketing safety of migraine prophylactic monoclonal antibodies: An EudraVigilance database analysis of eptinezumab, fremanezumab, galcanezumab, and erenumab., PMID:40439253
Efficacy and Safety of Eptinezumab in Episodic Cluster Headache: A Randomized Clinical Trial., PMID:40388178
Genetic migraine disorders and the response to calcitonin gene-related peptide antagonist treatment., PMID:40341526
A 24-week prospective, multicenter, real-world study on eptinezumab's effectiveness and safety in migraine prevention (EMBRACE II)., PMID:40332560
Safety, efficacy, and compliance of moderate-to-high dose eptinezumab and erenumab in chronic migraine patients with medication-overuse headache: an updated systematic review and meta-analysis., PMID:40329188
Increased infection risk in patients on preventive CGRP-targeting therapies- a meta-analysis and clinical effect assessment., PMID:40281424
Long-term safety, tolerability, and efficacy of eptinezumab in chronic cluster headache (CHRONICLE): an open-label safety trial., PMID:40252664
PACAP38-induced migraine attacks are independent of CGRP signaling: a randomized controlled trial., PMID:40229719
[Successful Management of Wearing-off effect with Eptinezumab: Lessons from a case with Chronic Migraine Refractory to Two Subcutaneous CGRP Antibodies]., PMID:40191903
Efficacy and safety of mAbs anti-CGRP/CGRP R (eptinezumab and erenumab) or atogepant in combination with onabotulinumtoxinA in refractory chronic migraine: a clinical trial protocol., PMID:40169386
Tracking Migraine Symptoms: A Longitudinal Comparison of Smartphone-Based Headache Diaries and Clinical Interviews., PMID:40137454
Agreement Between Parental Self-Reported Antiseizure Medication Use and Dispensed Prescription Records From a National Prescription Database., PMID:40134051
CROATIAN GUIDELINES FOR SPECIFIC PREVENTIVE TREATMENT OF MIGRAINE WITH MONOCLONAL ANTIBODIES TARGETING CALCITONIN GENE-RELATED PEPTIDE (CGRP) (EPTINEZUMAB, FREMANEZUMAB, AND GALCANEZUMAB) OR THE CGRP RECEPTOR (ERENUMAB)., PMID:40104234
Ubrogepant, erenumab, and eptinezumab antagonize positive inotropic effects of the calcitonin gene-related peptide in the isolated human atrium., PMID:40085216
Treatment Patterns and Healthcare Resource Utilization by Gender and Migraine Frequency in Adult Patients Receiving Galcanezumab Versus Standard of Care Preventive Medications Over 24 months: A United States Retrospective Claims Study., PMID:40046564
Review: An Update on CGRP Monoclonal Antibodies for the Preventive Treatment of Episodic Migraine., PMID:39998706
Heavy chain variants affect light and heavy chains assembly of monoclonal antibody expressed by Pichia pastoris., PMID:39992035
Health Technology Assessment: Evaluation of 8 CGRP-Targeted Therapy Drugs for the Treatment of Migraine., PMID:39991088
Shared Genes and Pathways in Ulcerative Colitis and Ankylosing Spondylitis: Functional Validation and Implications for Diagnosis., PMID:39925932
Prevention of Episodic Migraine Headache Using Pharmacologic Treatments in Outpatient Settings: A Clinical Guideline From the American College of Physicians., PMID:39899861
Switching between anti-CGRP monoclonal antibodies in migraine prophylaxis., PMID:39884968
Pharmacological differences and switching among anti-CGRP monoclonal antibodies: A narrative review., PMID:39825578
A case of sporadic hemiplegic migraine treated with eptinezumab: should we consider anti-CGRP antibodies for selected patients?, PMID:39745588
Impact of eptinezumab on work productivity beyond reductions in monthly migraine days: post hoc analysis of the DELIVER trial., PMID:39692817
Eptinezumab treatment was associated with longer interictal headache/migraine periods which corresponded to greater improvements in patient-reported quality of life measures., PMID:39666143
Benefit-risk assessment based on number needed to treat and number needed to harm: Atogepant vs. calcitonin gene-related peptide monoclonal antibodies., PMID:39558612
Long-term reductions in acute headache medication use after eptinezumab treatment in patients with migraine and prior preventive treatment failures: Post hoc analysis of the DELIVER randomized trial., PMID:39501727
Efficacy of eptinezumab in non-responders to subcutaneous monoclonal antibodies against CGRP and the CGRP receptor: A retrospective cohort study., PMID:39469839
Hypersensitivity to the CGRP Inhibitor Monoclonal Antibodies Galcanezumab, Erenumab, and Fremanezumab With Tolerance to Eptinezumab., PMID:39441051
Migraine treatment: Position paper of the French Headache Society., PMID:39406556
Comparative efficacy and safety of different pharmacological therapies to medication overuse headache: a network meta-analysis., PMID:39375607
Preventive drug treatments for adults with chronic migraine: a systematic review with economic modelling., PMID:39365169
First real-world study on the effectiveness and tolerability of rimegepant for acute migraine therapy in Chinese patients., PMID:39333875
The influence of pharmacodynamics and pharmacokinetics on the antimigraine efficacy and safety of novel anti-CGRPergic pharmacotherapies: a narrative review., PMID:39319681
Population pharmacokinetics of eptinezumab in paediatric patients with migraine and dose selection for phase 3 paediatric migraine studies., PMID:39206528
Advancing toward precision migraine treatment: Predicting responses to preventive medications with machine learning models based on patient and migraine features., PMID:39176658
Evaluating the Effectiveness, Tolerability, and Safety of Eptinezumab in High-Frequency and Chronic Migraine in Real World: EMBRACE-The First Italian Multicenter, Prospective, Real-Life Study., PMID:39061413
A Risk-Difference Meta-Analysis for the Prophylactic Treatments of Chronic Migraine., PMID:39022494
Long-term effectiveness of eptinezumab in the treatment of patients with chronic migraine and medication-overuse headache., PMID:38924044
Effectiveness and safety of pharmacological prophylaxis for chronic migraine: a systematic review and network meta-analysis., PMID:38910144
A real-world prospective observational study of eptinezumab in Asian patients with migraine., PMID:38785386
Effectiveness and tolerability of eptinezumab in treating patients with migraine resistant to conventional preventive medications and CGRP (receptor) antibodies: a multicentre retrospective real-world analysis from Germany., PMID:38755541
Real-world effectiveness and satisfaction with intravenous eptinezumab treatment in patients with chronic migraine: REVIEW, an observational, multi-site, US-based study., PMID:38664605
Adverse and serious adverse events incidence of pharmacological interventions for managing chronic and episodic migraine in adults: a systematic review., PMID:38646505
An economic evaluation of eptinezumab for the preventive treatment of migraine in the UK, with consideration for natural history and work productivity., PMID:38637754
Reporting Quality and Risk of Bias Analysis of Published RCTs Assessing Anti-CGRP Monoclonal Antibodies in Migraine Prophylaxis: A Systematic Review., PMID:38610729
Preventive Treatment of Migraine., PMID:38568488
The transition of medication overuse status by acute medication categories in episodic or chronic migraine patients to non-overuse status after receiving anti-CGRP monoclonal antibodies: a systematic review and meta-analysis of phase 3 randomized control trial., PMID:38564060