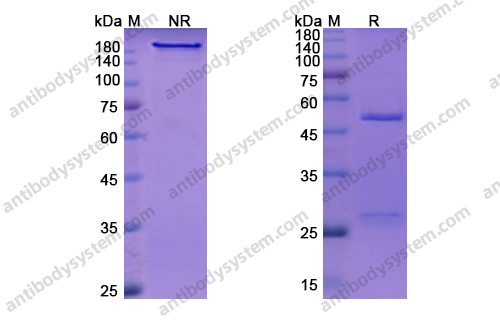
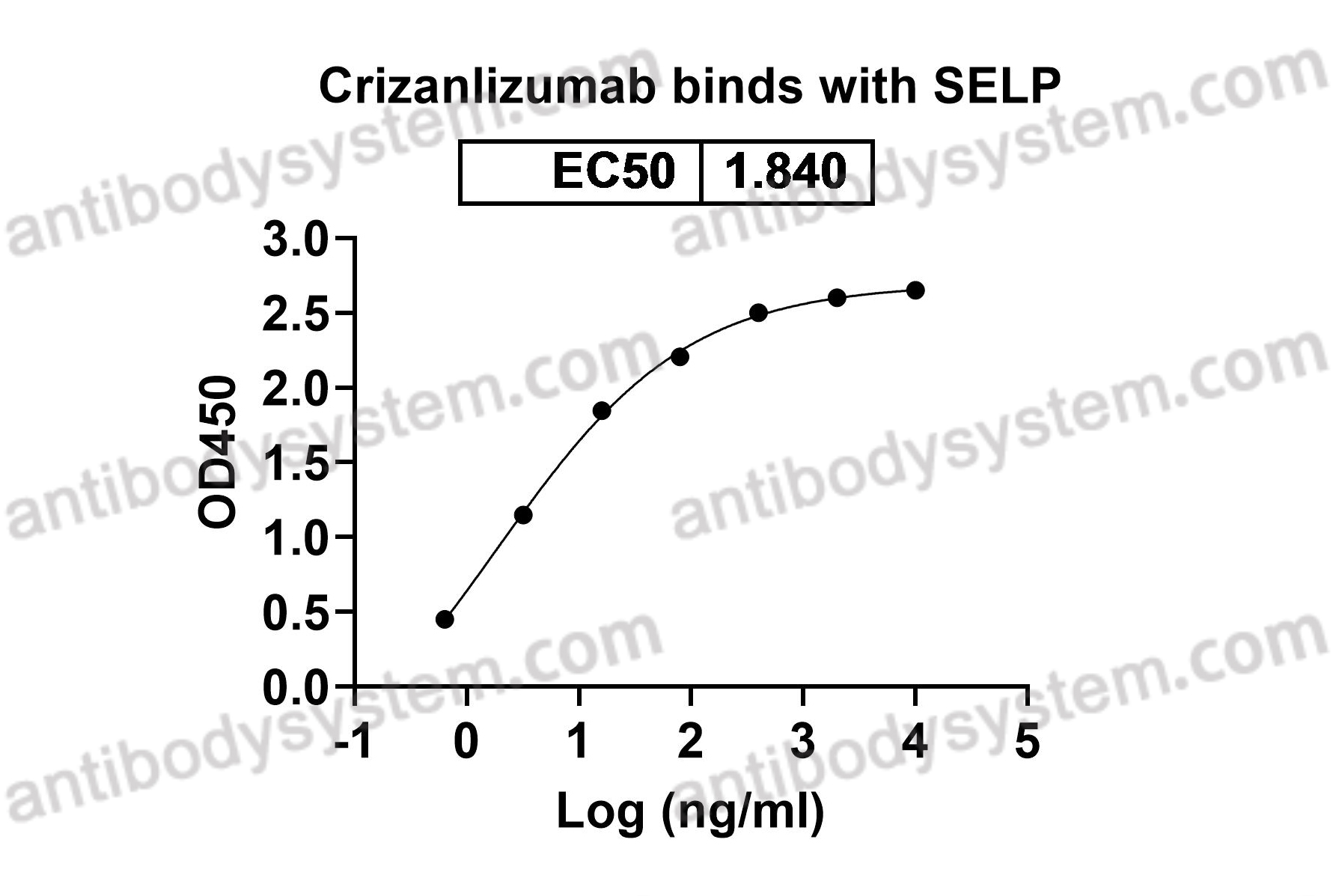
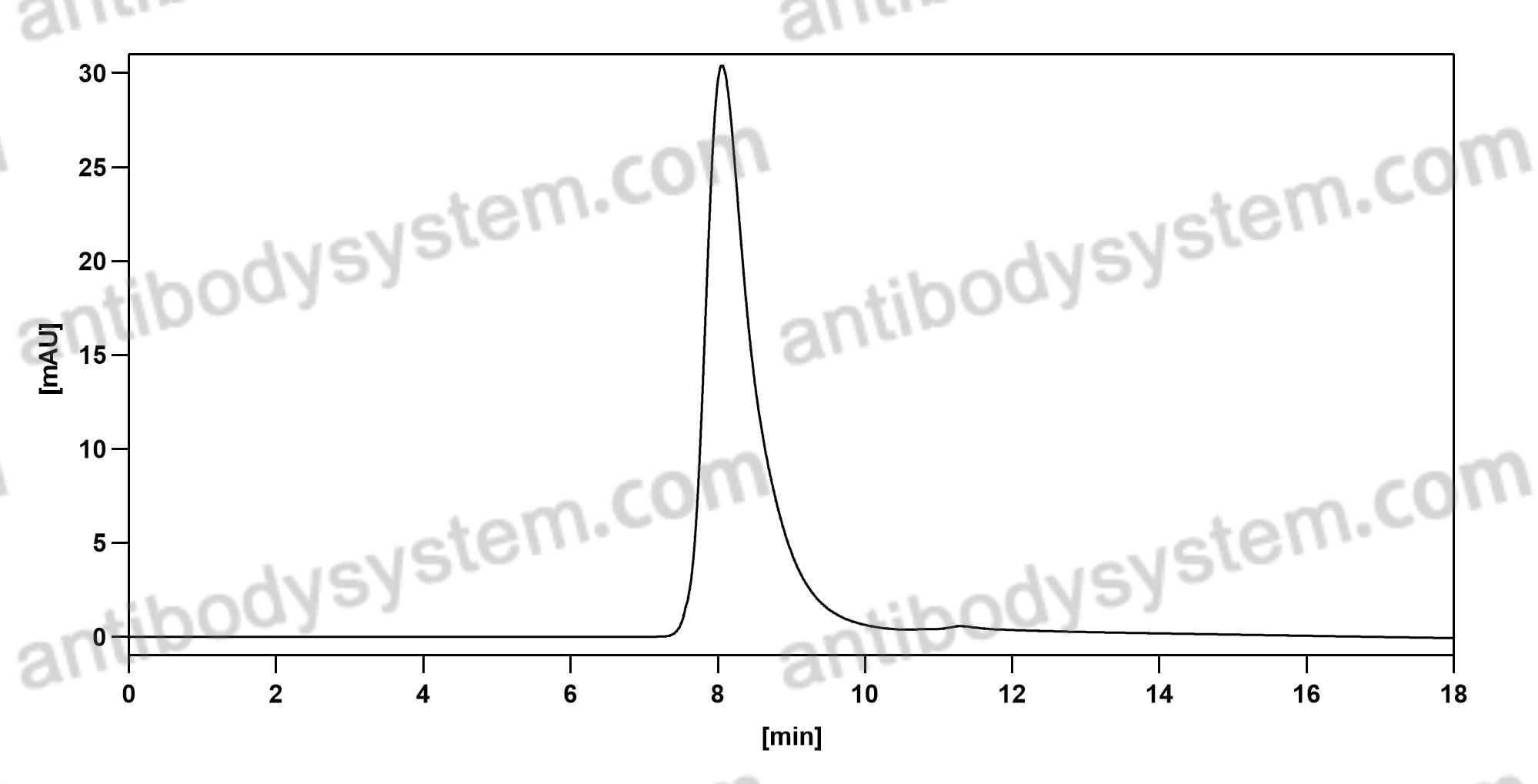
Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease, PMID: 27959701
Crizanlizumab: First Approval, PMID: 31933169
Effect of crizanlizumab on pain crises in subgroups of patients with sickle cell disease: A SUSTAIN study analysis, PMID: 30295335
Crizanlizumab, PMID: 32401467
Systematic Review of Crizanlizumab: A New Parenteral Option to Reduce Vaso-occlusive Pain Crises in Patients with Sickle Cell Disease, PMID: 32350885
Crizanlizumab and comparators for adults with sickle cell disease: a systematic review and network meta-analysis, PMID: 32948541
Antibodies to watch in 2020, PMID: 31847708
Crizanlizumab, PMID: 34406730
Crizanlizumab in vaso-occlusive crisis caused by sickle cell disease, PMID: 33332478
Efficacy and safety of recently approved drugs for sickle cell disease: a review of clinical trials, PMID: 32841705
The vaso-occlusive pain crisis in sickle cell disease: Definition, pathophysiology, and management, PMID: 32301178
Antibodies to watch in 2019, PMID: 30516432
Crizanlizumab in Sickle Cell Disease, PMID: 28467874
Drug Therapies for the Management of Sickle Cell Disease, PMID: 32765834
Crizanlizumab in Sickle Cell Disease, PMID: 28467873
Crizanlizumab Prevents Sickle Cell Pain Crises, PMID: 28245319
Current and novel therapies for the prevention of vaso-occlusive crisis in sickle cell disease, PMID: 33062233
Challenges in the Management of Sickle Cell Disease During SARS-CoV-2 Pandemic, PMID: 32873056
Profile of crizanlizumab and its potential in the prevention of pain crises in sickle cell disease: evidence to date, PMID: 31507334
Severe infusion-related reaction to crizanlizumab in an adolescent with sickle cell disease, PMID: 32945013
Leukocyte adhesion to P-selectin and the inhibitory role of Crizanlizumab in sickle cell disease: A standardized microfluidic assessment, PMID: 32208292
The European Medicines Agency Review of Crizanlizumab for the Prevention of Recurrent Vaso-Occlusive Crises in Patients With Sickle Cell Disease, PMID: 34235401
P-Selectin Blockade in the Treatment of Painful Vaso-Occlusive Crises in Sickle Cell Disease: A Spotlight on Crizanlizumab, PMID: 33833562
More Unnecessary Imaginary Worlds - Part 4: The ICER Evidence Report for Crizanlizumab, Voxelotor and L-Glutamine for Sickle Cell Disease, PMID: 34007618
P-selectin blockade in COVID-19-related ARDS, PMID: 32464083
The Evolving Pharmacotherapeutic Landscape for the Treatment of Sickle Cell Disease, PMID: 31934320
Two drugs for sickle cell disease, PMID: 32324178
Sickle Cell Disease: Advances in Treatment, PMID: 30559624
What are the key considerations when prescribing pharmacotherapy for sickle cell anemia?, PMID: 32955945
Managing patients with sickle cell disease in primary care, PMID: 32941305
Two New Drugs for Sickle Cell Disease, PMID: 32079792
Treating sickle cell anemia: A new era dawns, PMID: 31925819
Emerging pharmacotherapeutic approaches for the management of sickle cell disease, PMID: 30499731
Advances in Sickle Cell Disease Treatments, PMID: 32520675
Innovative Treatments for Rare Anemias, PMID: 34095760
Antibodies to watch in 2018, PMID: 29300693
Thrombin activation of PAR-1 contributes to microvascular stasis in mouse models of sickle cell disease, PMID: 31977004
Novartis snaps up Selexys for sickle cell, PMID: 28178263
Pharmacotherapeutical strategies in the prevention of acute, vaso-occlusive pain in sickle cell disease: a systematic review, PMID: 29296801
Research in Sickle Cell Disease: From Bedside to Bench to Bedside, PMID: 34095767
Curative vs targeted therapy for SCD: does it make more sense to address the root cause than target downstream events?, PMID: 32722787
Contemporary Management and Prevention of Vaso-Occlusive Crises (VOCs) in Adults With Sickle Cell Disease, PMID: 34151636
Elevated P-Selectin in Severe Covid-19: Considerations for Therapeutic Options, PMID: 33747397
Review of Medication Therapy for the Prevention of Sickle Cell Crisis, PMID: 30013299
Antiplatelet agents for preventing vaso-occlusive events in people with sickle cell disease: a systematic review, PMID: 31188815
Sickle cell vaso-occlusion: The dialectic between red cells and white cells, PMID: 33794696
ADORE: an open platform study of ruxolitinib in combination with other novel therapies in patients with myelofibrosis., PMID:40334082
Evidence and gaps in clinical outcomes of novel pharmacologic therapies for sickle cell disease: A systematic literature review highlighting insights from clinical trials and real-world studies., PMID:40307078
Clinically relevant clot resolution via a thromboinflammation-on-a-chip., PMID:40175551
Impact of Different Definitions of Vaso-Occlusion on Efficacy Assessments in Sickle Cell Disease Clinical Trials., PMID:40146367
Now where do we STAND with crizanlizumab?, PMID:40088923
Crizanlizumab with or without hydroxyurea in patients with sickle cell disease (STAND): primary analyses from a placebo-controlled, randomised, double-blind, phase 3 trial., PMID:40088922
Accelerated drug approvals and patient trust: impact of voxelotor and crizanlizumab for sickle cell disease., PMID:40085957
A critique review of fetal hemoglobin modulators through targeting epigenetic regulators for the treatment of sickle cell disease., PMID:40057227
A review on disease modifying pharmacologic therapies for sickle cell disease., PMID:39828781
A contemporary review of the management strategies for sickle cell disease related ischaemic and stuttering priapism., PMID:39709509
Crizanlizumab for retinal vasculopathy with cerebral leukoencephalopathy in a phase II clinical study., PMID:39680465
Practical guide for disease-modifying medication management of children and adolescents with sickle cell disease., PMID:39644011
Novel Pathway and Recent Advances for Targeting Sickle Cell Anemia through Novel Drug Delivery System., PMID:39629577
Targeting P-selectin and interleukin-1β in mice with sickle cell disease: effects on vaso-occlusion, liver injury and organ iron deposition., PMID:39568425
Promising role of voxelotor in managing sickle cell disease in children: a narrative review., PMID:39533724
The Current Role of Hydroxyurea in the Treatment of Sickle Cell Anemia., PMID:39518543
Pharmacokinetics, pharmacodynamics, safety, and efficacy of crizanlizumab in patients with sickle cell disease: final results from the phase II SOLACE-adults study., PMID:39497751
Real-World Evidence of Crizanlizumab Showing Reductions in Vaso-Occlusive Crises and Opioid Usage in Sickle Cell Disease., PMID:39473076
Gene Therapies for Sickle Cell Disease., PMID:39391326
Advancing life: innovative approaches to enhance survival in sickle cell anemia patients., PMID:39359845
Newer Modalities and Updates in the Management of Sickle Cell Disease: A Systematic Review., PMID:39286637
Budget Impact of Disease-Modifying Treatments and a CRISPR Gene-Edited Therapy for Sickle Cell Disease., PMID:39134876
Exploring the genetic mechanisms: SELP gene's contribution to alleviating vaso-occlusive crisis in sickle cell disease., PMID:39079562
Current and emerging drug treatment strategies to tackle sickle cell anemia., PMID:38988318
The enigma of sickle cell hepatopathy: Pathophysiology, clinical manifestations and therapy., PMID:38978231
Crizanlizumab for retinal vasculopathy with cerebral leukoencephalopathy in a phase II clinical study., PMID:38950286
Potential efficacy of crizanlizumab in treating priapism in sickle cell disease: A case report., PMID:38736574
Clinical Practice Patterns in Sickle Cell Disease Treatment: Disease-modifying and Potentially Curative Therapies., PMID:38718300
Cost-effectiveness of l-glutamine versus crizanlizumab for adults with sickle cell disease: model focused on reducing pain episode costs from Qatar's healthcare perspective., PMID:38711465
Sickle Cell Disease in Brazil: Current Management., PMID:38663998
Expert consensus on the management of infusion-related reactions (IRRs) in patients with sickle cell disease (SCD) receiving crizanlizumab: a RAND/UCLA modified Delphi panel., PMID:38642304
Spanish registry of hemoglobinopathies and rare anemias (REHem-AR): demographics, complications, and management of patients with β-thalassemia., PMID:38519604
Plant-Produced Therapeutic Crizanlizumab Monoclonal Antibody Binds P-Selectin to Alleviate Vaso-occlusive Pain Crises in Sickle Cell Disease., PMID:38491245
Crizanlizumab and sickle cell disease: When should medications have their approval status revoked?, PMID:38409818
Recurrent Nontraumatic Subgaleal Hematomas in a Pediatric Patient With Sickle Cell Disease., PMID:38408160
An update review of new therapies in sickle cell disease: the prospects for drug combinations., PMID:38344818
Casgevy and Lyfgenia: Two gene therapies for sickle cell disease., PMID:38212256
Real-world experience of patients with sickle cell disease treated with crizanlizumab., PMID:38073007
Using disease-modifying therapies in sickle cell disease., PMID:38066905
Use of Disease-Modifying Treatments in Patients With Sickle Cell Disease., PMID:37991760
Red Blood Cells as Therapeutic Target to Treat Sickle Cell Disease., PMID:37975291
Comparative pharmacovigilance assessment of adverse events associated with the use of hydroxyurea, L-glutamine, voxelotor, and crizanlizumab in sickle cell disease., PMID:37950855
Sickle cell disease: combination new therapies vs. CRISPR-Cas9 potential and challenges - review article., PMID:37867187
Crizanlizumab in sickle cell disease., PMID:37850353
Successful Treatment of SCD-Related Priapism With Crizanlizumab: A Case Series., PMID:37731262
Advancements in Sickle Cell Disease (SCD) Treatment: A Review of Novel Pharmacotherapies and Their Impact on Patient Outcomes., PMID:37664319
The Role of Non-genetic Therapies to Reduce the Incidence of Sickle Cell Crisis: A Systematic Review., PMID:37664256
P-Selectin de-ACTIVation in COVID-19: What Have We Learned?, PMID:37523764